A kind of preparation method of 2-chloro-5-iodobenzoic acid
A technology of iodobenzoic acid and o-chlorobenzoic acid, applied in the field of pharmaceutical synthesis, can solve the problems of high production cost, long reaction steps, low product yield and the like, and achieves improved iodine utilization, simple operation and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
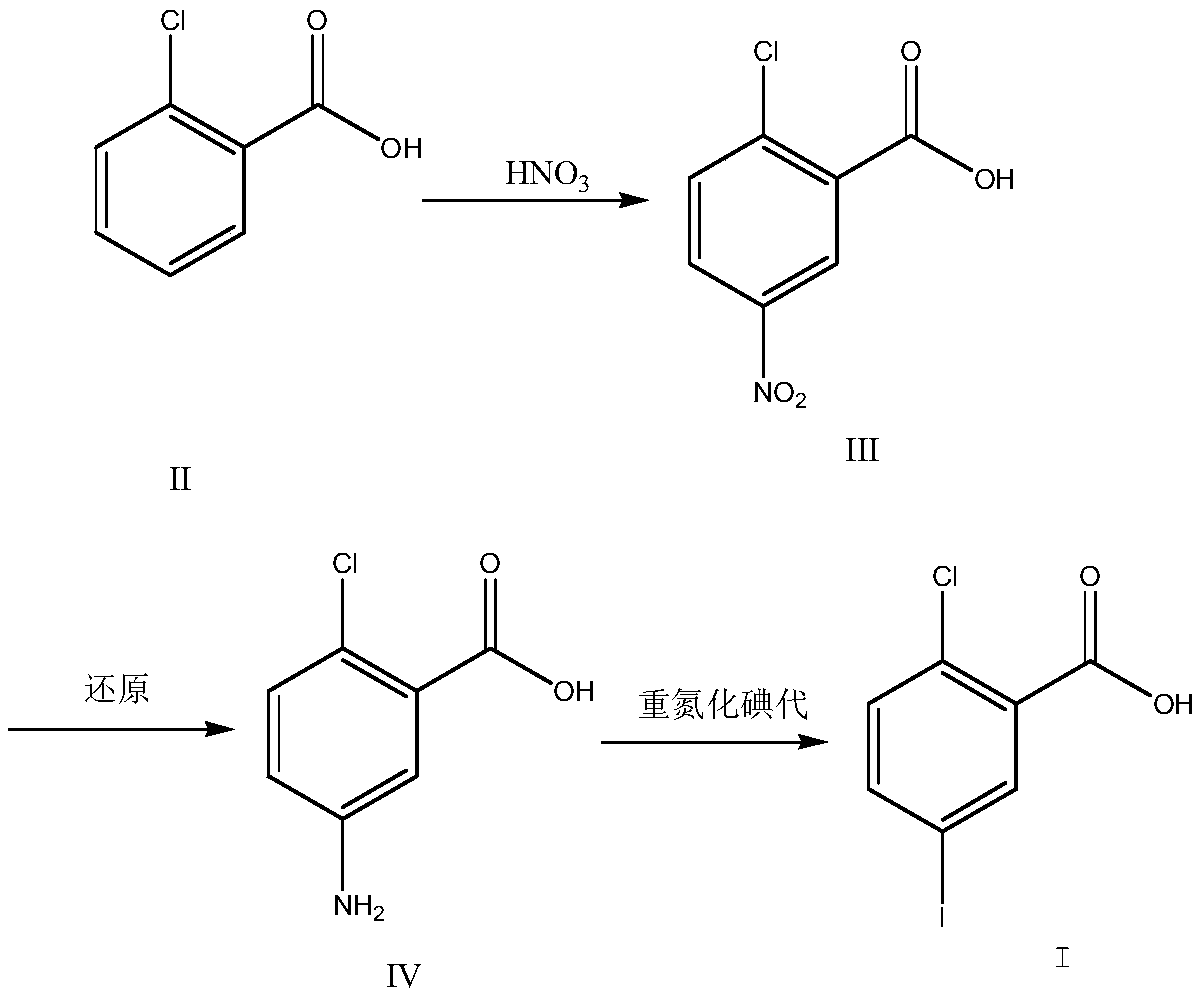
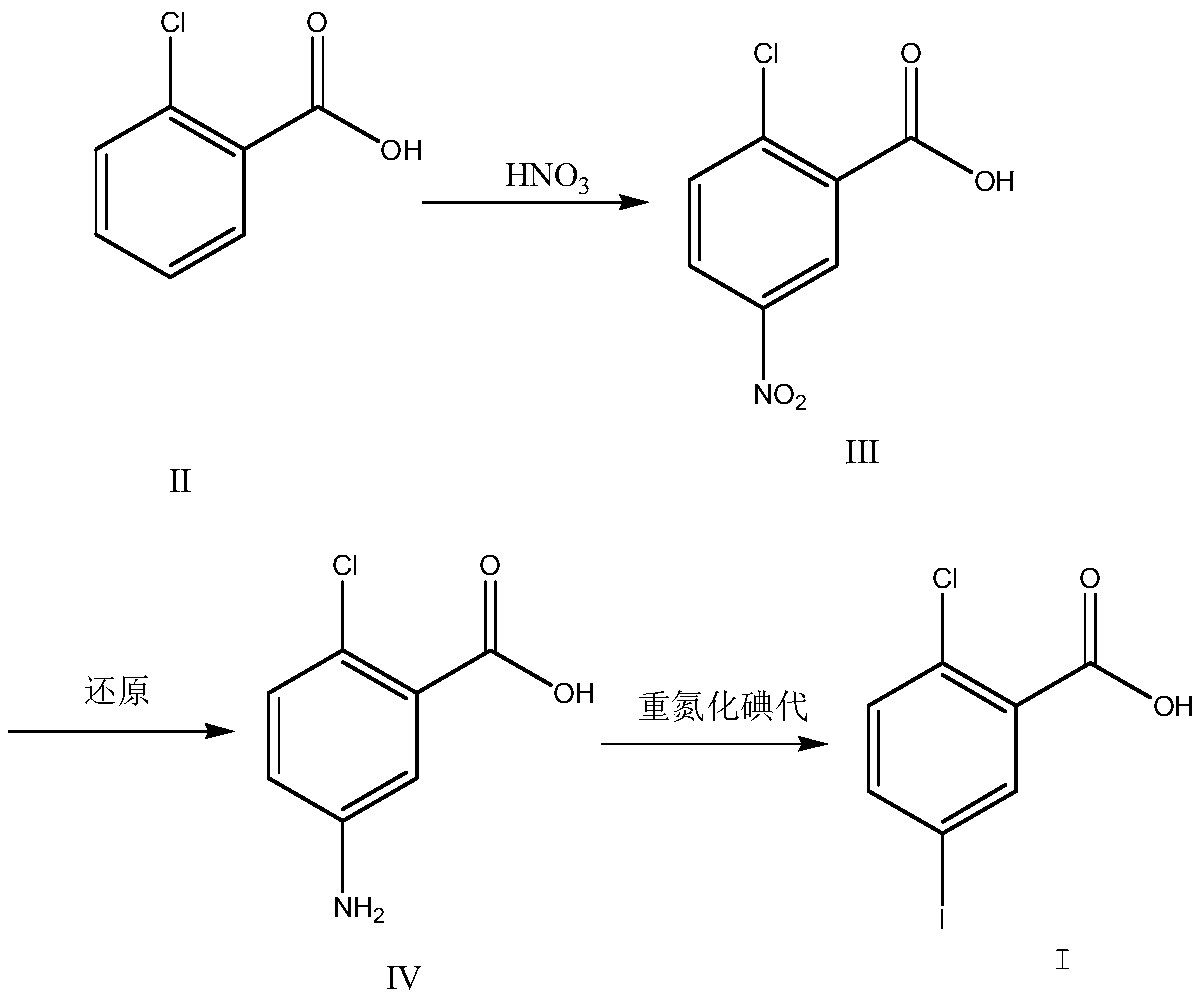
[0028] Put 196g of concentrated sulfuric acid into the reaction flask at room temperature, slowly add 31.2g of o-chlorobenzoic acid, stir until it dissolves; cool down to -5~0°C, add 21.6g of nitric acid (concentration 65%) dropwise, during the dropwise addition Temperature control -5~5°C; after dropping at 0~5°C for 2 hours, TLC (DCM:MeOH=10:1) to monitor the reaction, add 200ml of ice water dropwise to quench the reaction, control the temperature below 25°C, stir Centrifuge after 30 min, wash with 500 ml of water, and dry under reduced pressure at 50°C overnight to obtain 38.5 g of off-white solid 2-chloro-5-nitrobenzoic acid with a purity of 98.5% and a yield of 95.8%.
Embodiment 2
[0030] Add 75g of 2-chloro-5-nitrobenzoic acid, 59g of iron powder, 800ml of ethanol, 150ml of water, 115g of ammonium chloride into the reaction flask, heat to 78-80°C and reflux for 5h, TLC (PE / EA: 1 / 3) After monitoring the reaction, filter while it is hot, wash with 150ml of hot ethanol, evaporate the filtrate to dryness at 60°C to obtain the crude product, add 400ml of ethyl acetate, heat and reflux for beating for 2 hours, cool down to room temperature naturally, and then cool down to 0-5°C in a salt water bath to crystallize After more than 30 minutes, centrifuge, wash with 50ml of ethyl acetate, and dry under reduced pressure at 50°C to obtain 60.7g of 2-chloro-5-aminobenzoic acid as a yellow solid with a purity of 99.1% and a yield of 95.1%.
Embodiment 3
[0032] Add 123 g of 2-chloro-5-aminobenzoic acid into 2000 g of 20% sulfuric acid aqueous solution, keep stirring at 0-10°C, add dropwise an aqueous solution of sodium nitrite (51 g of sodium nitrite dissolved in 200 g of water), and stir until There is no solid in the reaction solution, TLC (PE / EA: 1 / 3) monitors after the reaction finishes, adds 1.2g urea, stirs and cools to 0 ℃, adds potassium iodide solution (130gKI is dissolved in 500g water) rapidly, rises to room temperature, stirs to Continue to stir for 30 minutes without bubbles, filter, wash with 200g of water to obtain a brown solid; dissolve the solid with 400g of ethyl acetate, wash with 300ml of 1N hydrochloric acid, 300ml of 10% sodium bisulfate, and 400ml of saturated brine, dry over magnesium sulfate, and reduce pressure at 50°C Dry to obtain the crude product, add 400ml of toluene at 80°C for beating for 1 hour, cool down at 0-5°C for crystallization for 1 hour, suction filter and dry at 50°C under reduced pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com