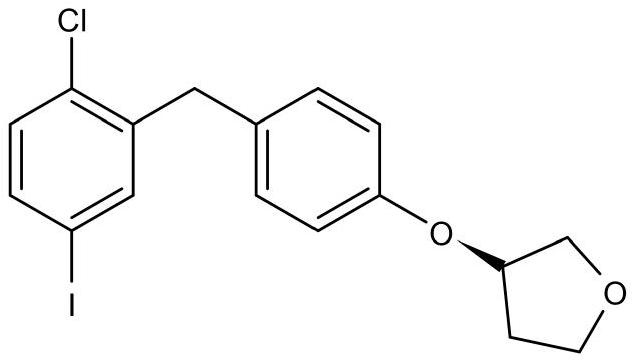
Synthetic method of empagliflozin intermediate
A technology of intermediates and mixed solvents, which is applied in the field of synthesis of empagliflozin intermediate-3-[4-[methyl]phenoxy]tetrahydrofuran, which can solve the problems of low yield, short synthetic route and unfavorable industrial production and other problems, to achieve the effect of high yield, short reaction steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
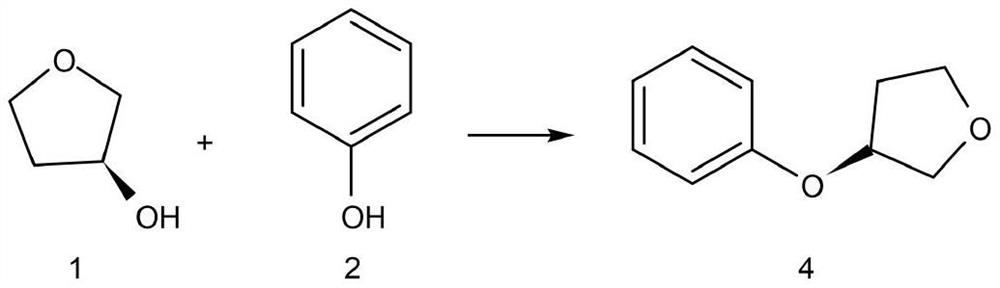
[0036] Step 1) Preparation of (S)-3-phenoxytetrahydrofuran
[0037] In THF (200 mL) were added phenol (4.70 g, 50 mmol), BINAP (32.4 g, 52 mmol) and (R)-3-hydroxytetrahydrofuran (4.58 g, 52 mmol), and MTAD (5.65 g, 50 mmol) was added dropwise. The solution was stirred overnight at room temperature (reaction completion monitored by HPLC). After the reaction, 6.90 g of (S)-3-phenoxytetrahydrofuran was obtained after filtration, concentration, extraction and purification, with a yield of 84.6% and a purity of 99.5%.
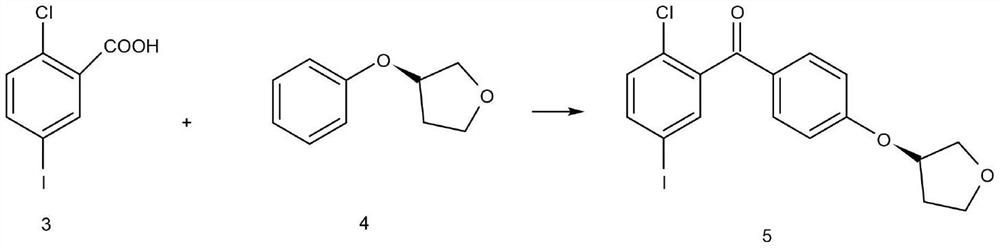
[0038] Step 2) Preparation of 2-3(5-iodo-2-chlorophenyl)[4-[[(3S)-tetrahydro-3-furyl]oxy]phenyl]methanone
[0039] In 150ml of tetrahydrofuran and dimethyl sulfoxide mixed solution (ratio 2:1), add 2-chloro-5-iodobenzoic acid (11.30g, 40mmol) and (S)-3-phenoxytetrahydrofuran (6.57 g, 40mmol), then TsCl (0.3g, 1.6mmol) was added, stirred at room temperature, slowly added dropwise 8ml EDTA, TLC showed that the reaction was completed after 1h, at room temperature the...
Embodiment 2
[0043] Step 1) Preparation of (S)-3-phenoxytetrahydrofuran
[0044]In THF (300 mL) were added phenol (7.05 g, 75 mmol), BINAP (46.73 g, 75 mmol) and (R)-3-hydroxytetrahydrofuran (6.61 g, 75 mmol), and MTAD (8.48 g, 75 mmol) was added dropwise. The solution was stirred overnight at room temperature (reaction completion monitored by HPLC). After the reaction was completed, 10.53 g of (S)-3-phenoxytetrahydrofuran was obtained after filtration, concentration, extraction and purification, with a yield of 84.6% and a purity of 99.5%.
[0045] Step 2) Preparation of 2-3(5-iodo-2-chlorophenyl)[4-[[(3S)-tetrahydro-3-furyl]oxy]phenyl]methanone
[0046] In 240ml of tetrahydrofuran and dimethyl sulfoxide mixed solution (ratio 2:1), add 2-chloro-5-iodobenzoic acid (16.95g, 60mmol) and (S)-3-phenoxytetrahydrofuran (9.86 g, 60mmol), then add TsCl (0.4g, 2.1mmol), stir at room temperature, slowly add dropwise 10ml EDTA, TLC shows that the reaction is completed after 1h, at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com