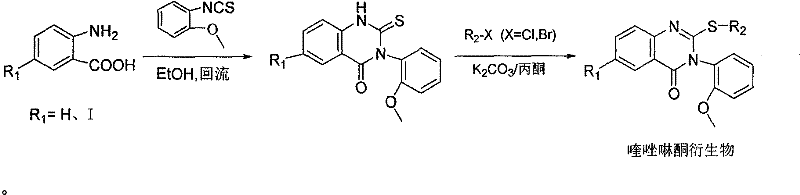
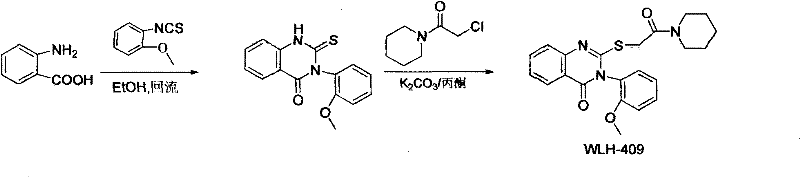
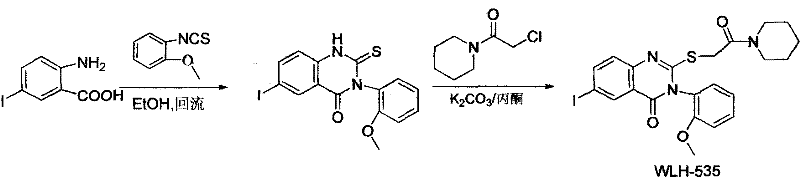
Quinazolinone derivatives and their preparation and application
A technology for quinazolinone and derivatives, applied in the fields of quinazolinone derivatives and their preparation and application, can solve problems such as being difficult to become drug candidate compounds, and achieve the effects of good pharmacokinetic properties and druggability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of WB-409
[0026]
[0027] In the first step, 8.6g of 2-amino-5-iodobenzoic acid and 10.4g of 2-methoxyphenylthioisocyanate were dissolved in 50mL of absolute ethanol, refluxed for 10 hours, cooled to room temperature, filtered, and the filter cake was washed with 10mL Petroleum ether rinse, 10mL saturated sodium bicarbonate solution rinse, 10mL anhydrous EtOH rinse, dry to obtain the quinazolinone intermediate crude product, 200-300 mesh silica gel column chromatography purification, eluent is dichloromethane and The mixed solvent of methyl alcohol, mixed solvent ratio is dichloromethane: methyl alcohol=100: 1, gets 5.4g quinazolinone intermediate, productive rate 30%, the compound 1 H NMR (DMSO, 500MHz): δ = 13.11 (brs, 1H), 8.18 (d, J = 2.0Hz, 1H), 8.05 (dd, J = 2.0, 4.2Hz, 1H), 7.39-7.42 (m, 1H ), 7.23(m, J=9.0Hz, 1H), 7.21(dd, J=1.5, 7.5Hz, 1H), 7.14(d, J=8.0Hz, 1H), 7.02(t, J=8.0Hz, 1H ), 3.71(s, 3H);
[0028] In the second step, the ...
Embodiment 2
[0029] Example 2: Preparation of WLH-535
[0030]
[0031] In the first step, 16.6g of anthranilic acid and 10.4g of 2-methoxyphenyl thioisocyanate were dissolved in 50mL of absolute ethanol, refluxed for 10 hours, cooled to room temperature, filtered, and the filter cake was rinsed with 10mL of petroleum ether , 10mL saturated sodium bicarbonate solution rinse, 10mL anhydrous EtOH rinse, dry to obtain the quinazolinone intermediate crude product, 200-300 mesh silica gel column chromatography purification, eluent is a mixed solvent of dichloromethane and methanol , the mixed solvent ratio is dichloromethane: methyl alcohol=100: 1, obtains 6.7g quinazolinone intermediate, productive rate 26%, the compound 1 H NMR (DMSO, 500MHz): δ = 13.11 (brs, 1H), 8.18 (d, J = 2.0Hz, 1H), 8.05 (dd, J = 2.0, 4.2Hz, 1H), 7.39-7.42 (m, 1H ), 7.23(d, J=9.0, 1H), 7.21(dd, J=1.5, 7.5Hz, 1H), 7.14(d, J=8.0Hz, 1H), 7.02(t, J=7.5Hz, 1H) , 3.71(s, 3H);
[0032] In the second step, the quinazolino...
Embodiment 3
[0034] Example Three: Preparation of WLH-424
[0035] In the second step, the halogenating reagent is Add-on is 0.14g, productive rate is 65%, the derivative 1 H NMR (CDCl 3 , 500MHz): δ=11.52(brs, 1H), 8.27(d, J=5.0Hz, 1H), 7.82(d, J=5.0Hz, 2H), 7.54(t, J=10.0Hz, 1H), 7.47 (t, J=15.0Hz, 1H), 7.43(m, 1H), 7.25(s, 1H), 7.09(t, J=10.0Hz, 1H), 6.98-6.97(s, 1H), 4.02(d, J=15Hz, 1H), 3.89(d, J=15.0Hz, 1H), 3.82(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com