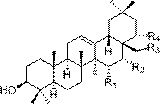
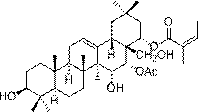
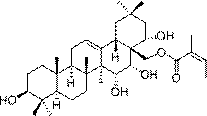
Barrigenol-like triterpenoids, and preparation method and pharmaceutical application thereof
A kind of technology of triterpene compound, uricol, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: prepare described triterpenoid compound
[0028] 3.5 kg of red bark rough fruit tea ( Camellia crapnelliana ) branches and leaves were dried and pulverized, extracted 6 times with 6 L of 90% methanol at room temperature, each time for 24 hours, combined the extracts, concentrated and evaporated methanol to obtain a total extract of 237 g. After the total extract was dispersed with 1.2L of water, it was extracted three times with equal volumes of petroleum ether, ethyl acetate, and n-butanol, respectively, to obtain 2 g of petroleum ether, 13.1 g of ethyl acetate, and 99.3 g of n-butanol. Water 4 components. The ethyl acetate fraction (13.1 mg) was eluted through the MCI column with a gradient of methanol-water (50: 50 - 70: 30 - 85: 15 - 100: 0, v / v), and were combined into 7 groups according to the color development of TLC Divided Fr.1 - Fr.7. Fr.6 (3.1 g) was packed on a silica gel column and eluted with PE-EtOAc gradient (10:1 - 5:1 -1:1, v / v) to obt...
Embodiment 2
[0042] Example 2: Determination of protein tyrosine phosphatase 1B inhibitory activity
[0043] Experimental method: Using molecular biology methods to construct genetically recombined hGST-PTP1B-BL21 E. coli Human PTP1B engineering bacteria, the purified hGST-PTP1B recombinant protein can hydrolyze the substrate para The phospholipid bond of -Nitrophenyl Phosphate(pNPP), the obtained dephosphorylated product pNP has a strong light absorption at a wavelength of 405 nm, so the change of the light absorption at 405 nm can be directly detected to observe the change of the enzyme activity and the effect of the compound on the enzyme activity Inhibition of PTP1B enzyme activity when the concentration of the compound was selected as 100 μM for primary screening, the test results showed that the inhibition rates of compounds 1-4 were 94.14%, 94.24%, 95.67% and 89.05% respectively ; Further determination of IC 50 Value: the sample is dissolved in DMSO to make a suitable concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com