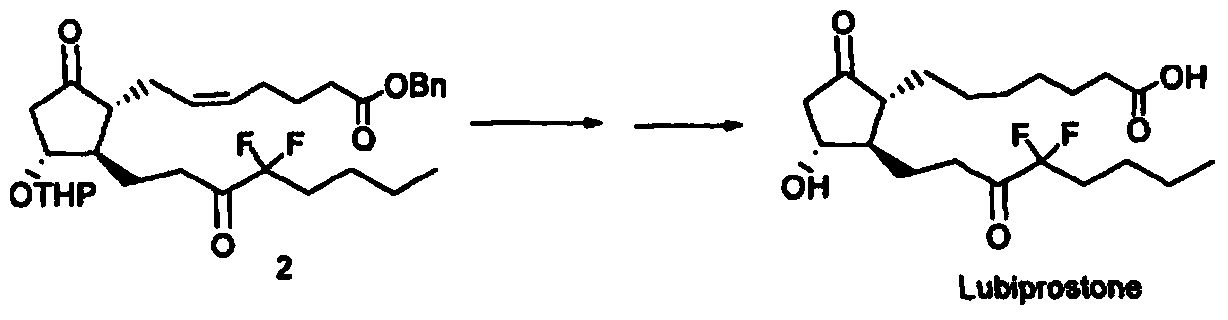Preparation method for prostaglandin intermediate
A preparation step, lubiprostone technology, applied in the field of preparation of prostaglandin intermediates, can solve serious, difficult to control, environmental pollution and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The method for preparing the compound of formula I provided by the present invention includes the following steps:
[0035] In the first step, the solution 2 containing the compound represented by formula II and the solution 3 containing the compound represented by formula III are mixed at 0-10°C, and then stirred at 0-30°C; the solution 2 or solution 3 contains The solvent is dichloromethane;
[0036] In the second step, after adding the aqueous solution, back-extract with dichloromethane for 1-3 times, and combine the dichloromethane layers; and
[0037] In the third step, the obtained dichloromethane layer solution was subjected to silica gel chromatography to obtain the compound represented by formula I.
[0038]
[0039] Preferably, in the first step, the solution 2 containing the compound represented by formula II can be added dropwise to the solution 3 containing the compound represented by formula III at 0-10°C, and then reacted at 0-30°C; or The solution 3 of the comp...
Embodiment 1
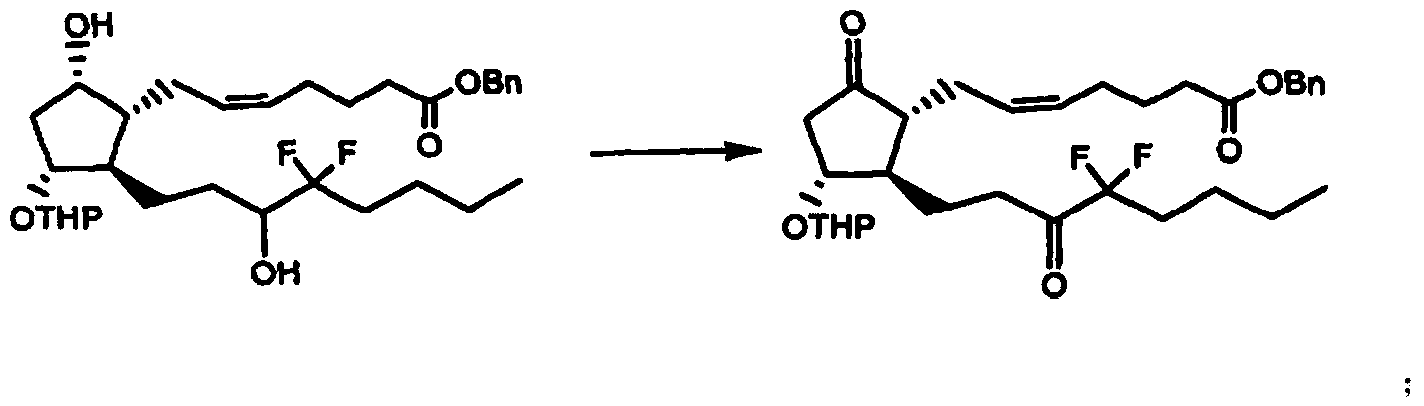
[0056] Preparation of 16,16-difluoro-13,14-dihydro-15-carbonyl-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGE 2 Methyl ester 1
[0057]
[0058] At room temperature, stir Dess-Martin oxidant (105.7g) and dissolve it in dichloromethane (1300ml), N 2 Under protection, cool to 0℃, add dropwise 16,16-difluoro-13,14-dihydro-15(R,S)-hydroxy-11-[(tetrahydro-2H-pyran-2-yl)oxy ]-PGF 2 A solution of methyl ester (54.3g) in dichloromethane (340ml) was dripped in 20min. Raise to 10°C and stir for 5h. TLC detected that the reaction was complete.
[0059] A 1.3L aqueous solution of 377.6 g of sodium thiosulfate pentahydrate and 139.8 g of sodium bicarbonate was slowly added. Stir for 5 min, separate the layers, extract the water layer with 200ml of dichloromethane, combine the dichloromethane layers, wash with 1.5L of water, dry with anhydrous magnesium sulfate, and concentrate to dry to obtain a light yellow oil, which is passed through a silica gel column (purchased from Qingdao Ocean Chemical, (30...
Embodiment 2
[0061] Preparation of 16,16-difluoro-13,14-dihydro-15-carbonyl-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGE 2 Benzyl ester 2
[0062]
[0063] At room temperature, add 16,16-difluoro-13,14-dihydro-15(R,S)-hydroxy-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGF 2a Benzyl ester (54.3g) dissolved in dichloromethane (340ml), N 2 Under protection, cool to 5°C, stir and dissolve Dess-Martin oxidant (157.7g) in dichloromethane (1300ml), drip into the above benzyl ester solution, and finish dripping for 20 minutes. Raise to 20°C and stir for 1h. TLC detected that the reaction was complete.
[0064] A 1.3L aqueous solution of 377.6 g of sodium thiosulfate pentahydrate and 139.8 g of sodium bicarbonate was slowly added. Stir for 5 min, separate the layers, extract the water layer with 200ml of dichloromethane, combine the dichloromethane layers, wash with 1.5L of water, dry with anhydrous magnesium sulfate, and concentrate to dry to obtain a light yellow oil, which is passed through a silica gel column (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com