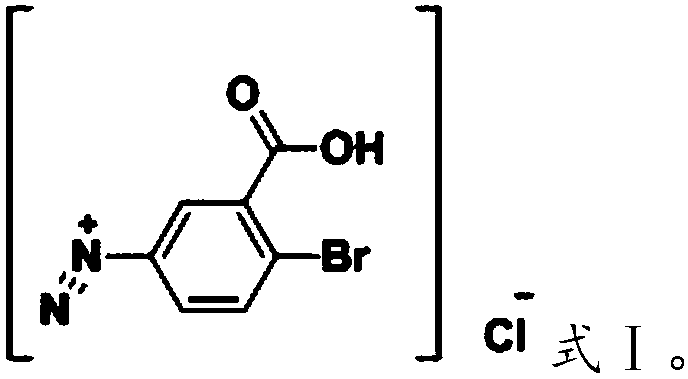
Preparation method of 2-bromo-5-iodobenzoic acid
A technology of bromobenzoic acid and iodobenzoic acid, applied in the field of preparation of 2-bromo-5-iodobenzoic acid, can solve problems such as only total yield, unsuitable for industrialized production, etc., achieves simple steps, good market prospects, and reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a kind of preparation method of 2-bromo-5-iodobenzoic acid, comprises the steps:
[0029] (1) 5-amino-2-bromobenzoic acid, inorganic acid, organic solvent and water are mixed to obtain 5-amino-2-bromobenzoic acid mixed solution;
[0030] (2) adding the nitrite aqueous solution dropwise to the 5-amino-2-bromobenzoic acid mixed solution to undergo a diazonium reaction to obtain a diazonium salt system;
[0031] (3) Add the iodide aqueous solution dropwise to the diazonium salt system to generate iodine reaction;
[0032] (4) After the iodination reaction is completed, an aqueous solution of sodium bisulfite is added to the system for quenching to obtain 2-bromo-5-iodobenzoic acid.
[0033] The invention mixes 5-amino-2-bromobenzoic acid, inorganic acid, organic solvent and water to obtain 5-amino-2-bromobenzoic acid mixed liquid. In the present invention, the mass ratio of the 5-amino-2-bromobenzoic acid, inorganic acid, organic solvent and water ...
Embodiment 1
[0058] Add 200.0 g of m-aminobenzoic acid and 760.0 g of N,N-dimethylformamide in turn into the reaction flask. Stir and cool down to 0°C; slowly add 273.0g of N-bromosuccinimide, control the temperature at 0°C, and complete the addition within 1h. Incubate at 0°C for 1 h. After the reaction was completed, 3.4Kg of water was added dropwise to quench the reaction. After the drop was completed, stirring was continued at 10° C. for 1 h. Filter and wash the filter cake once with 400.0 g of water. The wet product was dried at 60°C for 10 h to obtain 261.0 g of 5-amino-2-bromobenzoic acid with a product yield of 82.8%.
[0059] The obtained 5-amino-2-bromobenzoic acid is carried out to NMR and mass spectrometry detection, the results are as follows:
[0060] 1 H NMR (400MHz, DMSO) δ13.05(s), 7.26(d, J=8.6Hz), 6.94(d, J=2.8Hz), 6.59(dd, J=8.6, 2.8Hz), 5.52(s) .
[0061] MS calcd for C 7 h 7 BrNO 2 (M+H) + :217,found:217.
[0062] NMR and mass spectrometric detection result...
Embodiment 2
[0069] 20.0Kg of m-aminobenzoic acid and 76.0Kg of N,N-dimethylacetamide were successively added into the reaction kettle. Stir and cool down to -10°C; slowly add 27.3Kg of liquid bromine, control the temperature at -10°C, and complete the addition in 2 hours. The reaction was incubated at -10°C for 2 hours. After the reaction was completed, 340.0Kg of water was slowly sucked in to quench the reaction, and after the dripping was completed, stirring was continued at 0°C for 1 hour. Centrifuge, and wash the filter cake once with 20.0Kg water. The wet product was dried at 60°C for 12 hours to obtain 25.6Kg of 5-amino-2-bromobenzoic acid with a yield of 81.3%.
[0070] The obtained 5-amino-2-bromobenzoic acid was detected by NMR and mass spectrometry, and the results showed that the target product 5-amino-2-bromobenzoic acid was obtained.
[0071] 37.5Kg of 98% sulfuric acid, 163.0Kg of water, 163.0Kg of tetrahydrofuran and 25.0Kg of prepared 5-amino-2-bromobenzoic acid were su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com