Patents
Literature
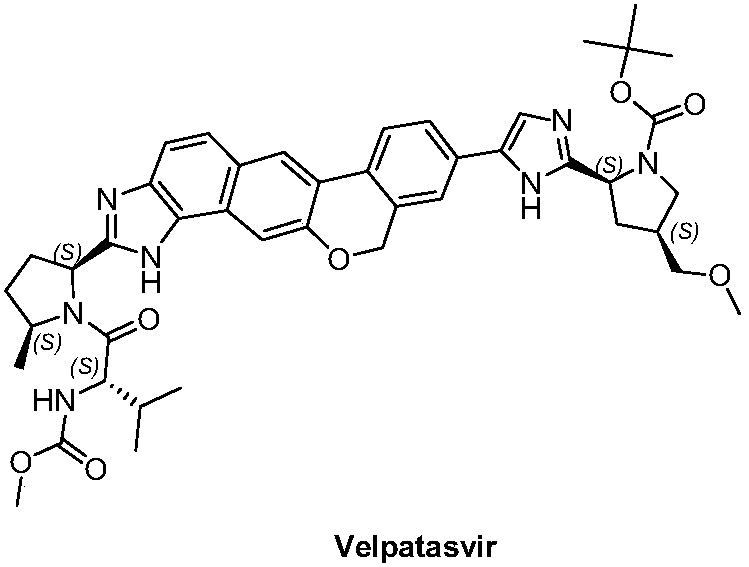
36 results about "Velpatasvir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
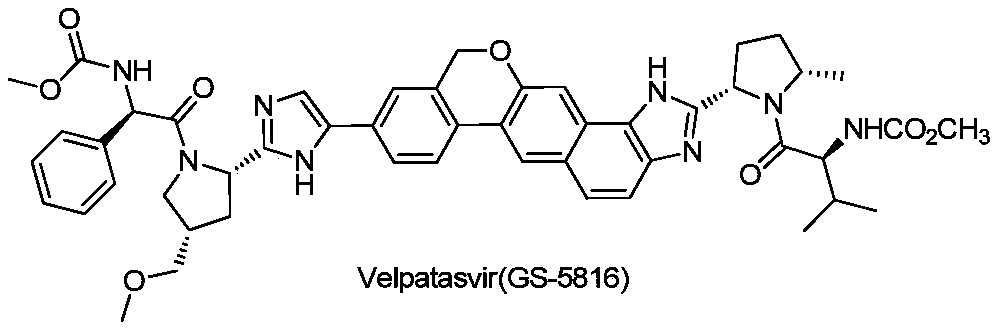
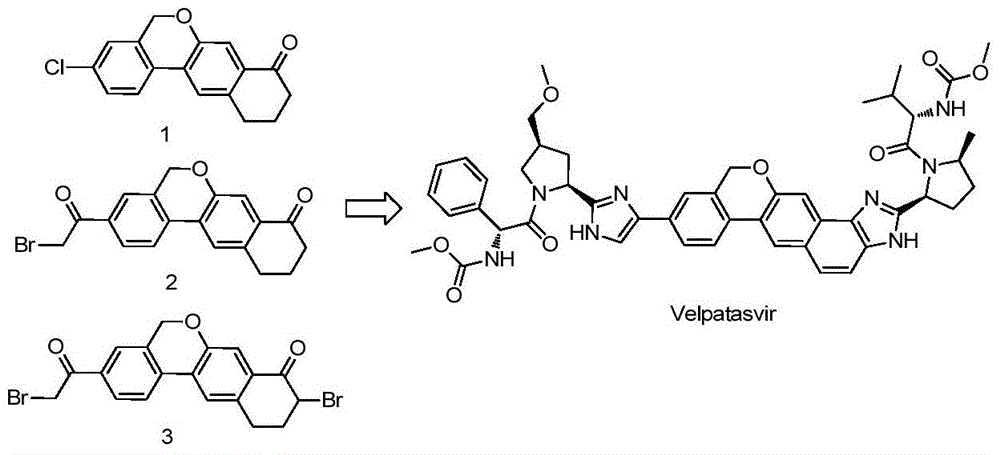
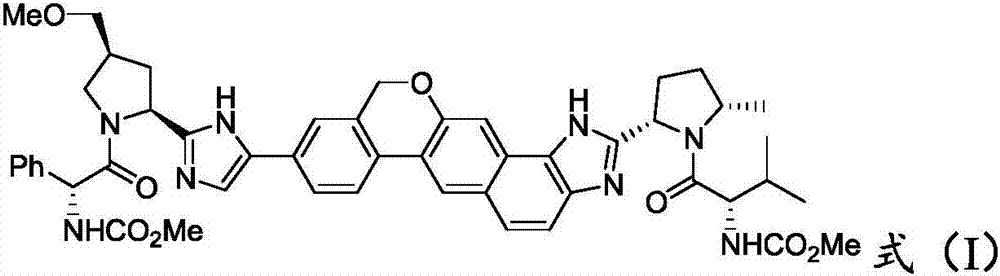
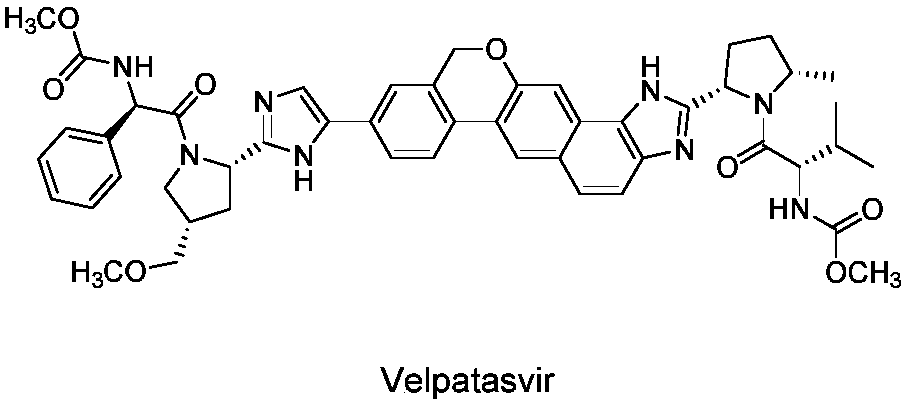
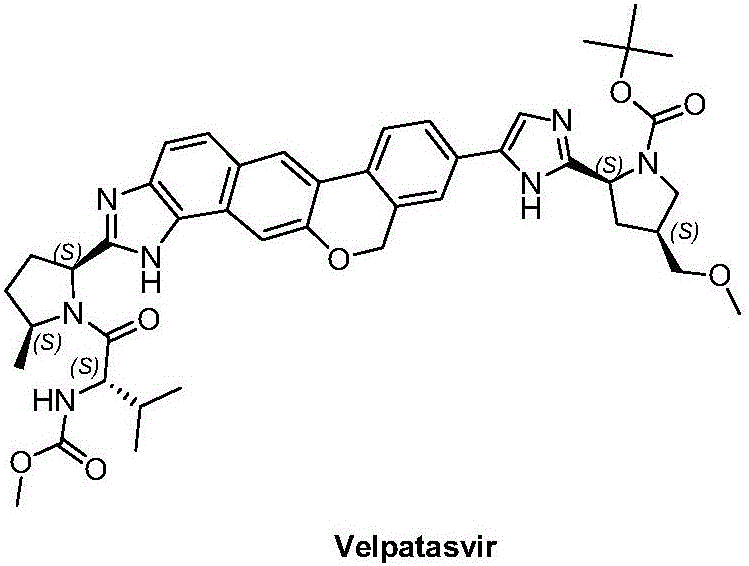
Velpatasvir is an NS5A inhibitor (by Gilead) which is used together with sofosbuvir in the treatment of hepatitis C infection of all six major genotypes.
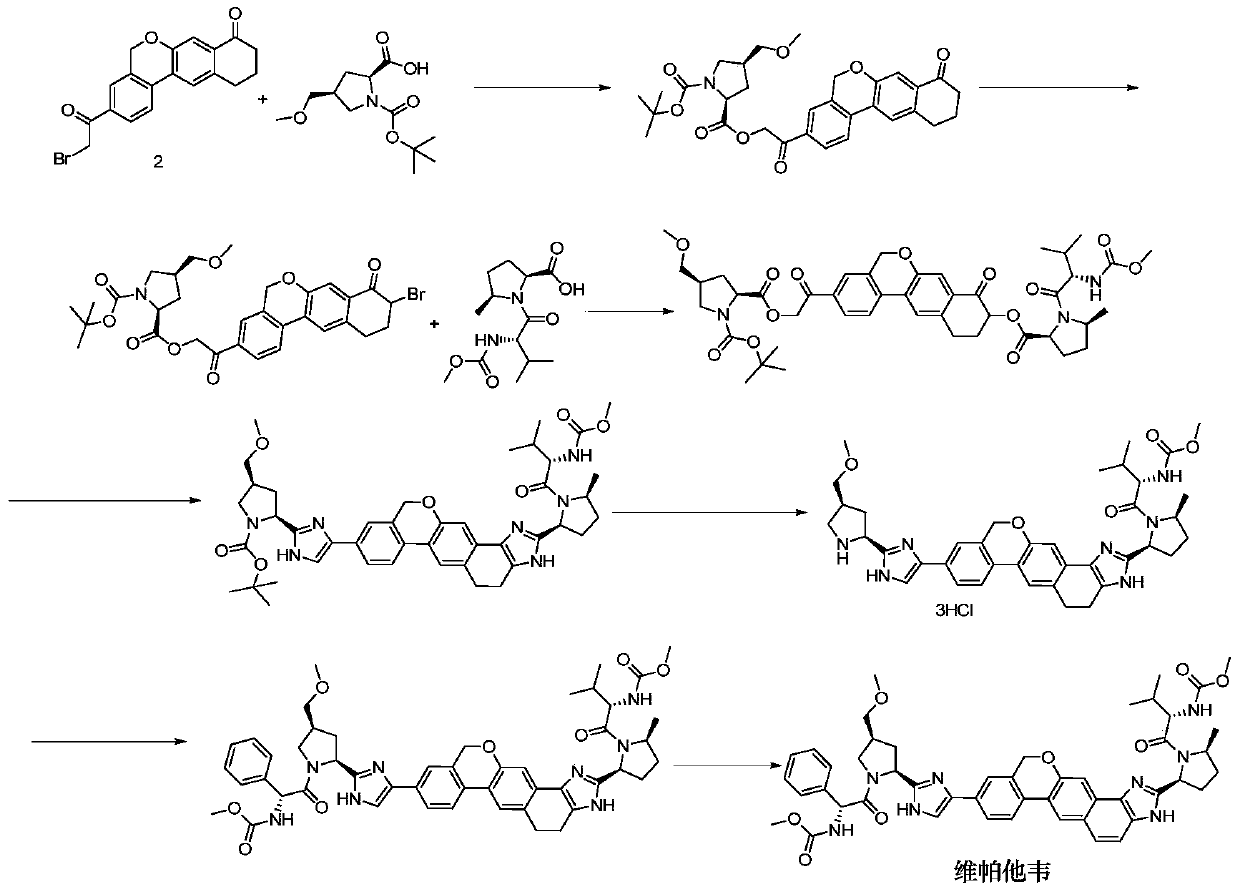
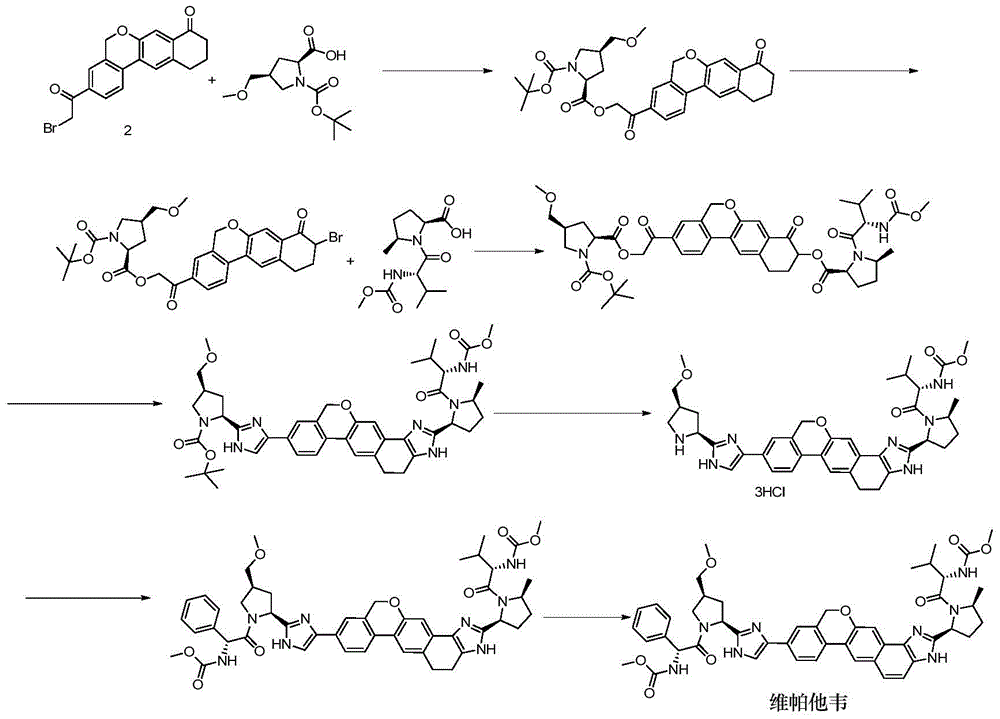
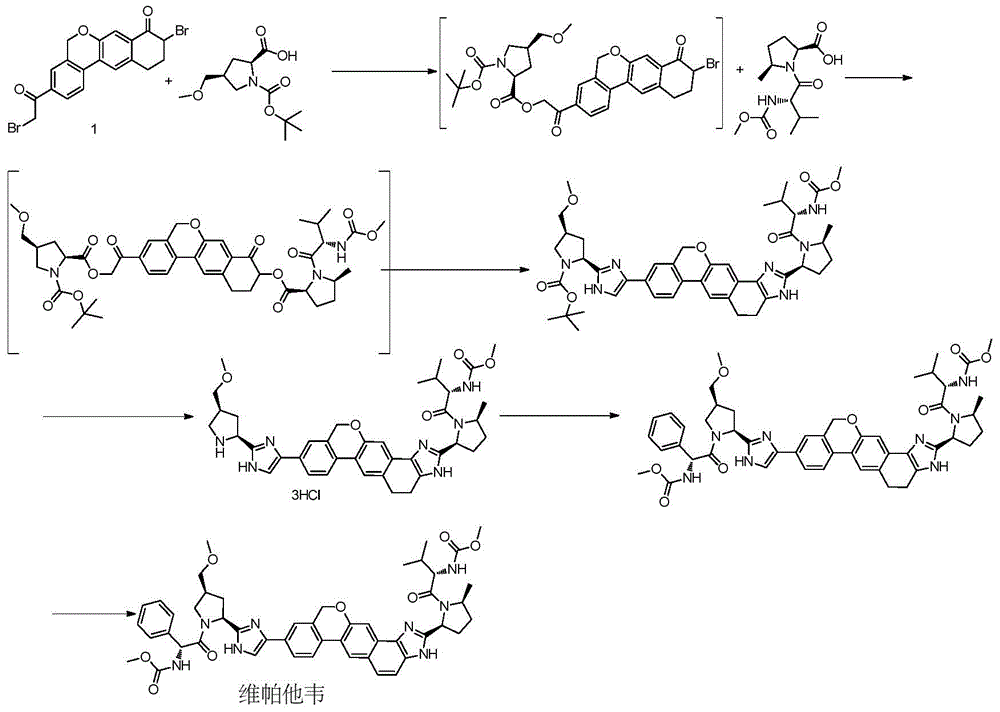
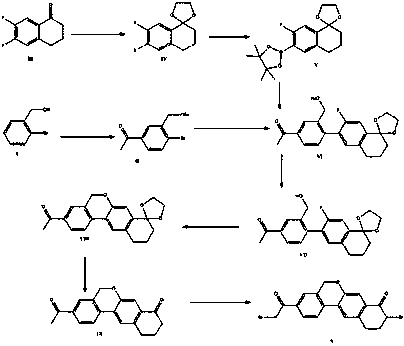
Novel synthesis method of hepatitis drug velpatasvir
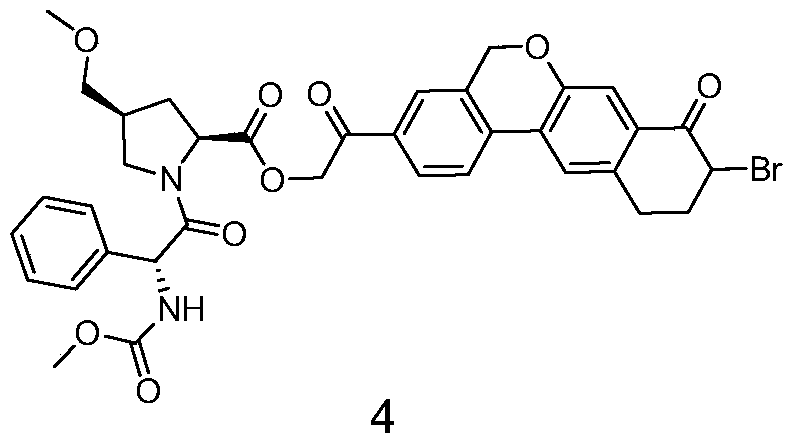
ActiveCN105732765AIncrease profitEfficient synthesisPeptidesTert-Butyloxycarbonyl protecting groupSynthesis methods
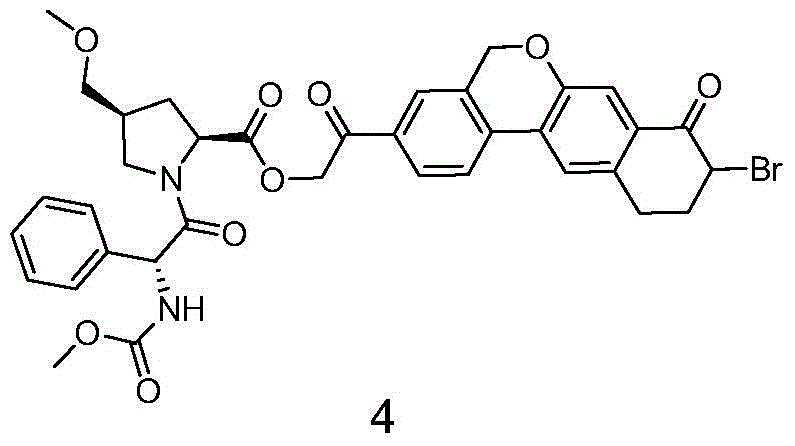
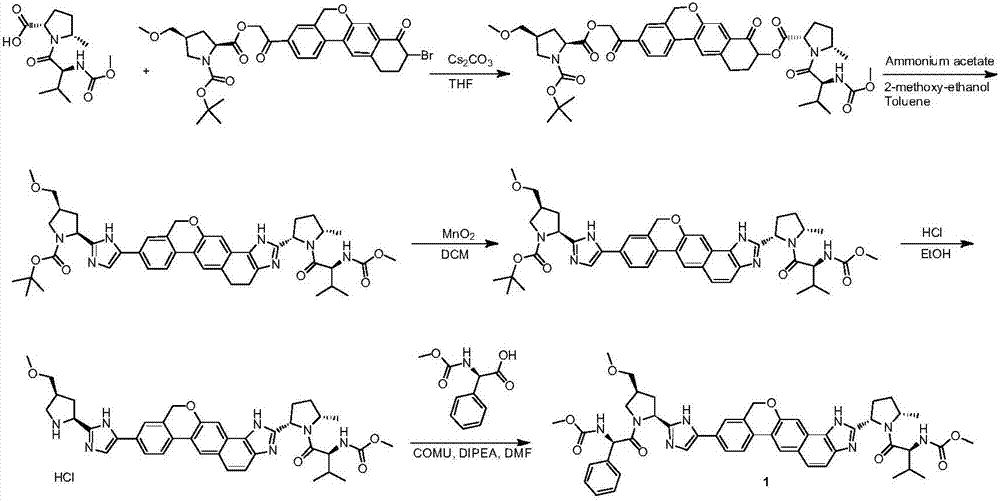
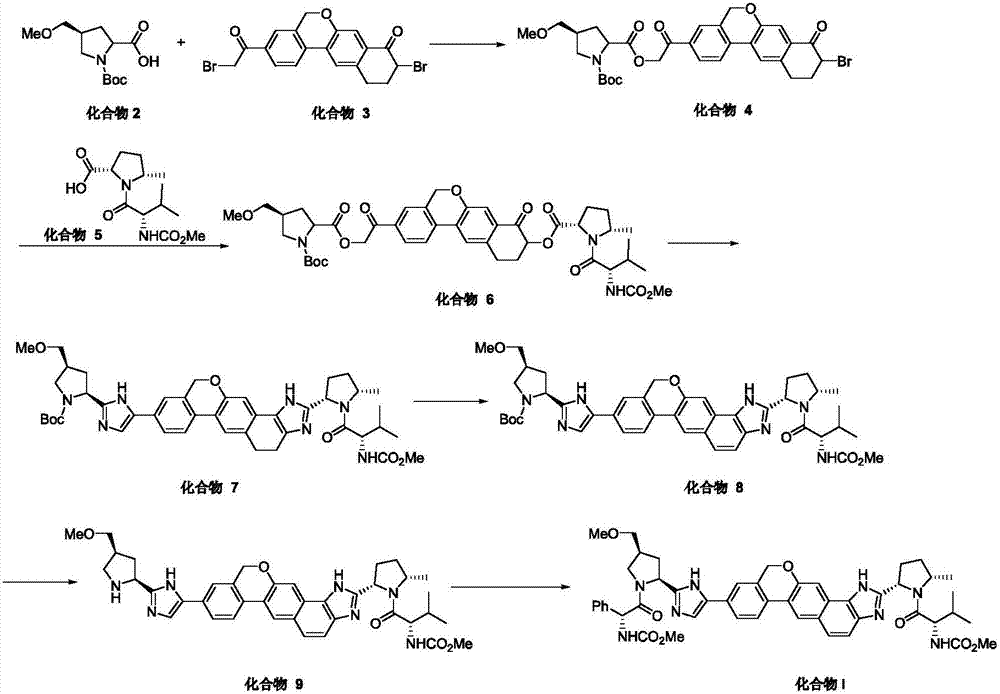
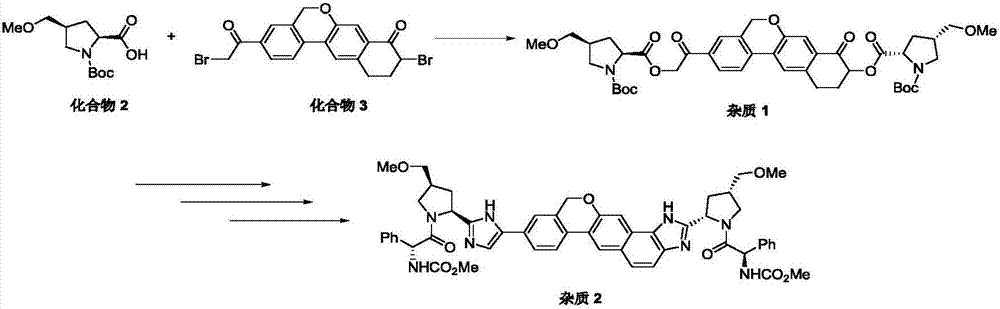
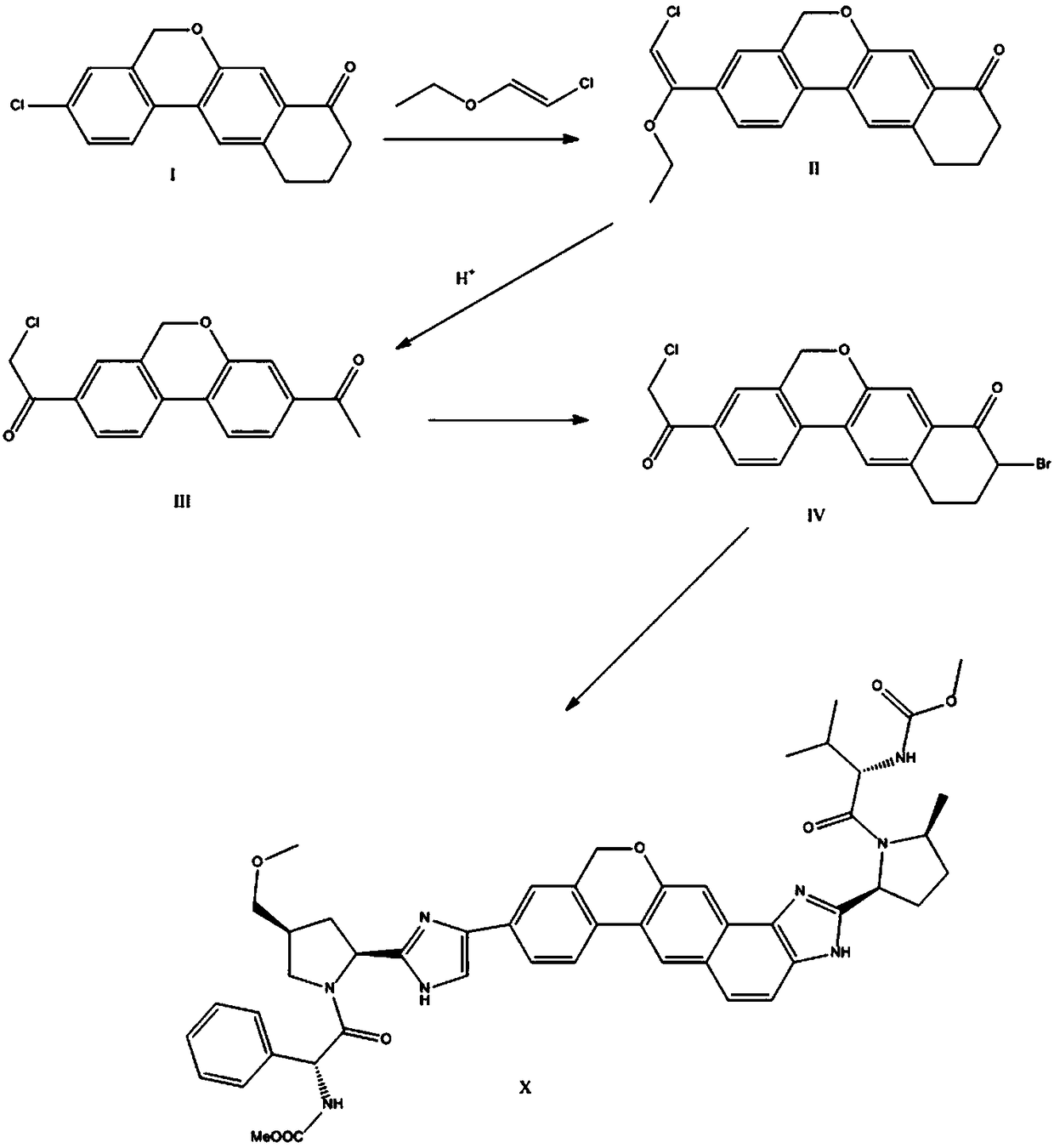
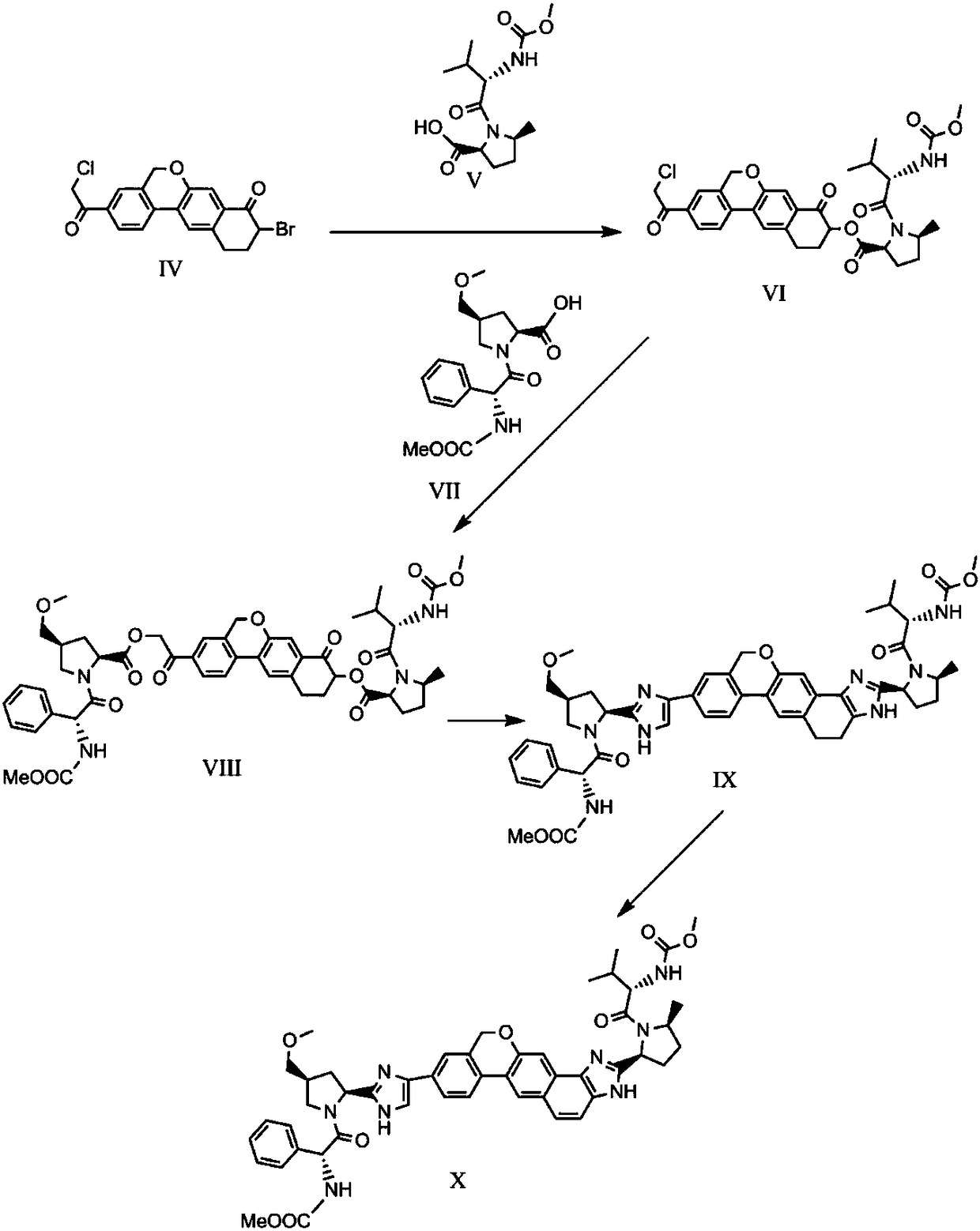
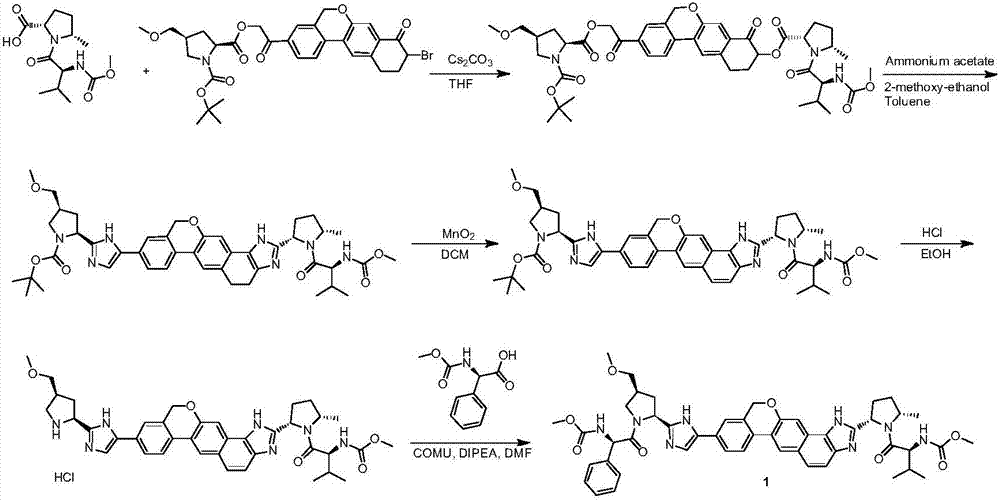
The invention provides a novel synthesis method of hepatitis drug velpatasvir.Two intermediate compounds including a compound 4 and a compound III are utilized to synthesize the velpatasvir, the structures of the two compounds are as shown in the following formulas, wherein PG radical is t-butyloxycarboryl (Boc), carboxybenzyl (Cbz), acetyl, benzoyl or (S)-2-methoxyl acyl carbonyl amino-3-methyl-butyryl group (Moc-L-Valyl).
Owner:山东科巢生物制药有限公司
Velpatasvir intermediate and preparation method thereof
The invention relates to a preparation method of a Velpatasvir (GS-5816) intermediate compound which serves as a hepatitis C resisting compound and is as shown in a formula 1, as well as preparation methods of a compound as shown in a formula 3 and a compound as shown in a formula 9. The compound as shown in the formula 3 and the compound as shown in the formula 9 are used for preparing a compound as shown in a formula 2 by nucleophilic substitution under the alkaline conditions; the compound as shown in the formula 2 is used for preparing the compound as shown in the formula 1 by a coupling reaction in the presence of a metal catalyst.
Owner:CONSCI PHARMA
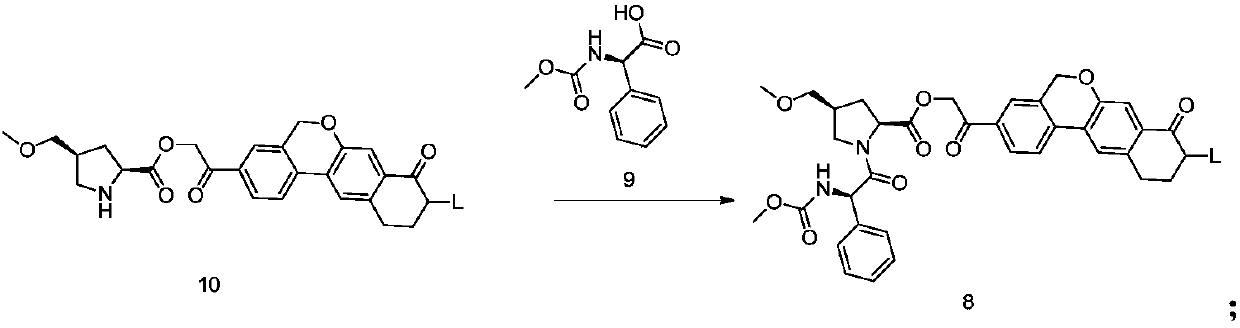
Preparation method of velpatasvir intermediate
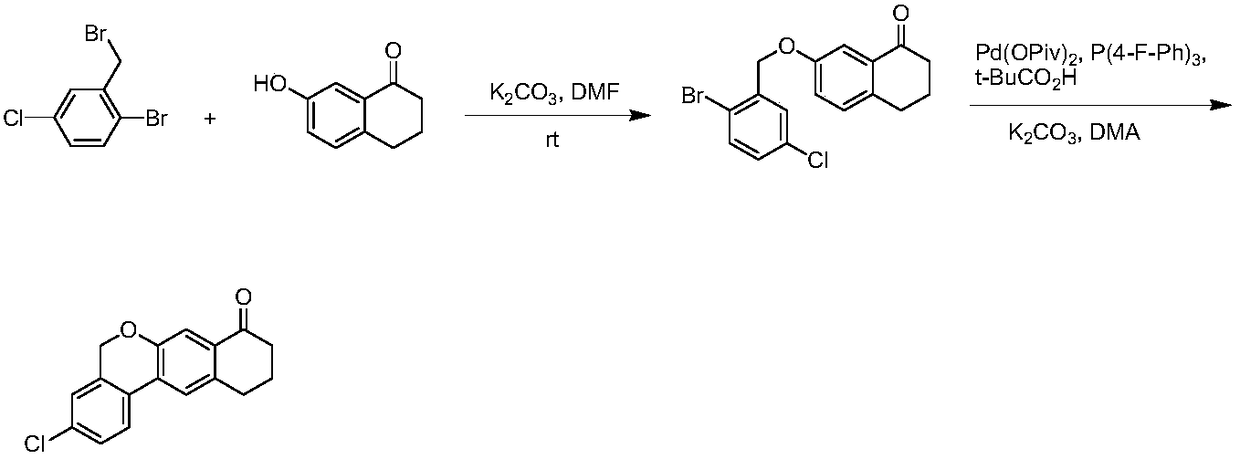
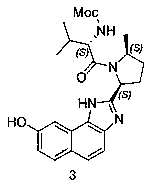
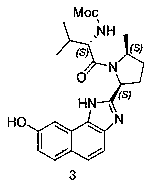
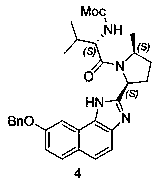
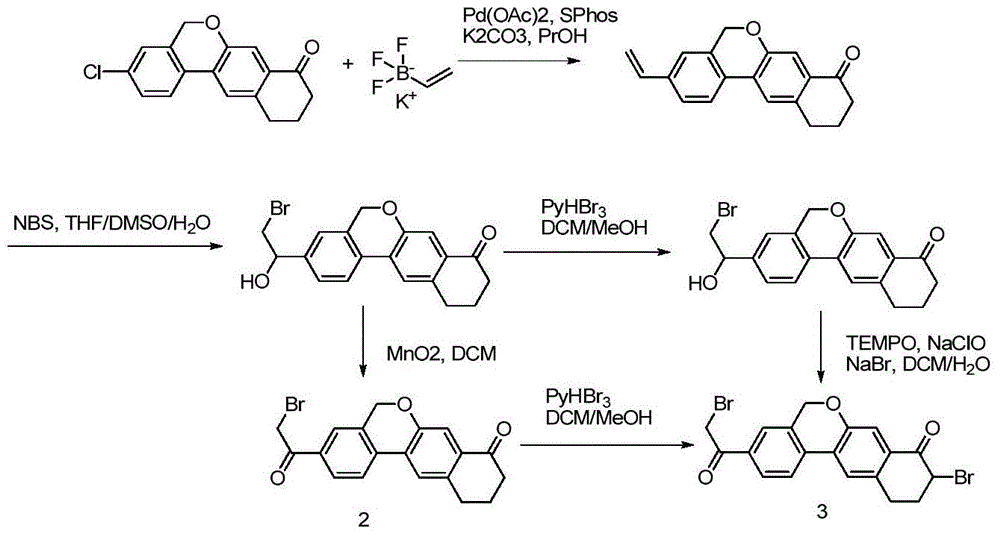
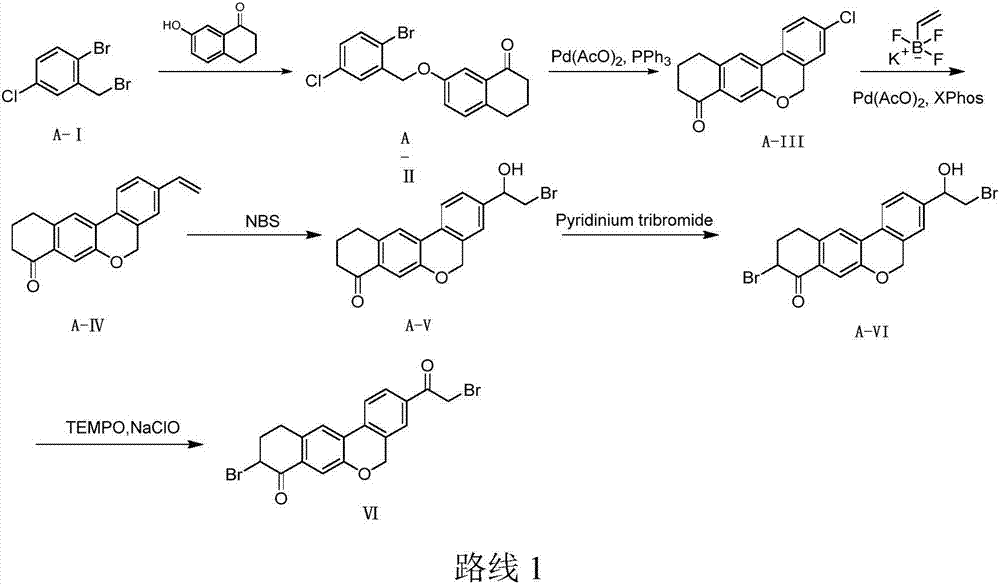
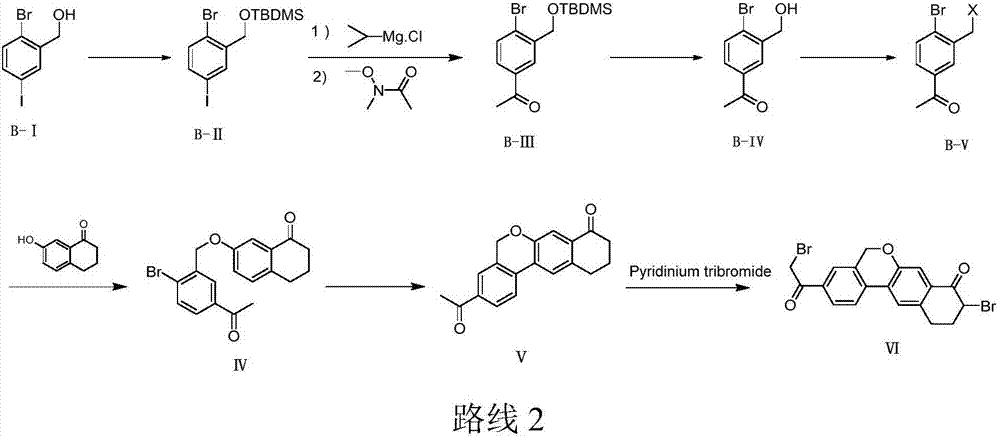
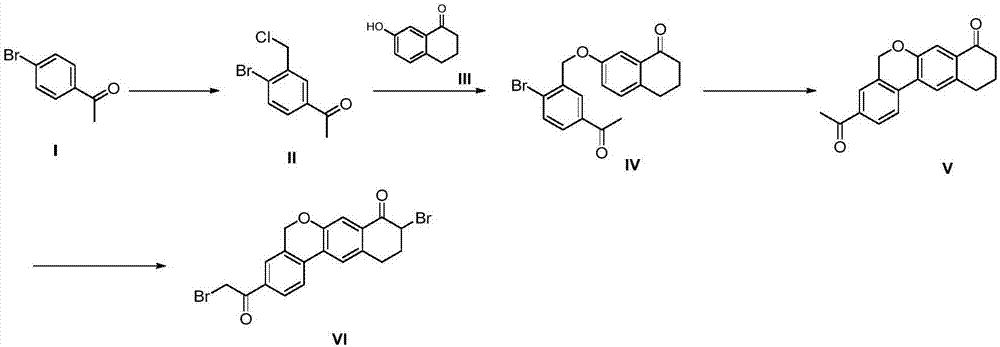
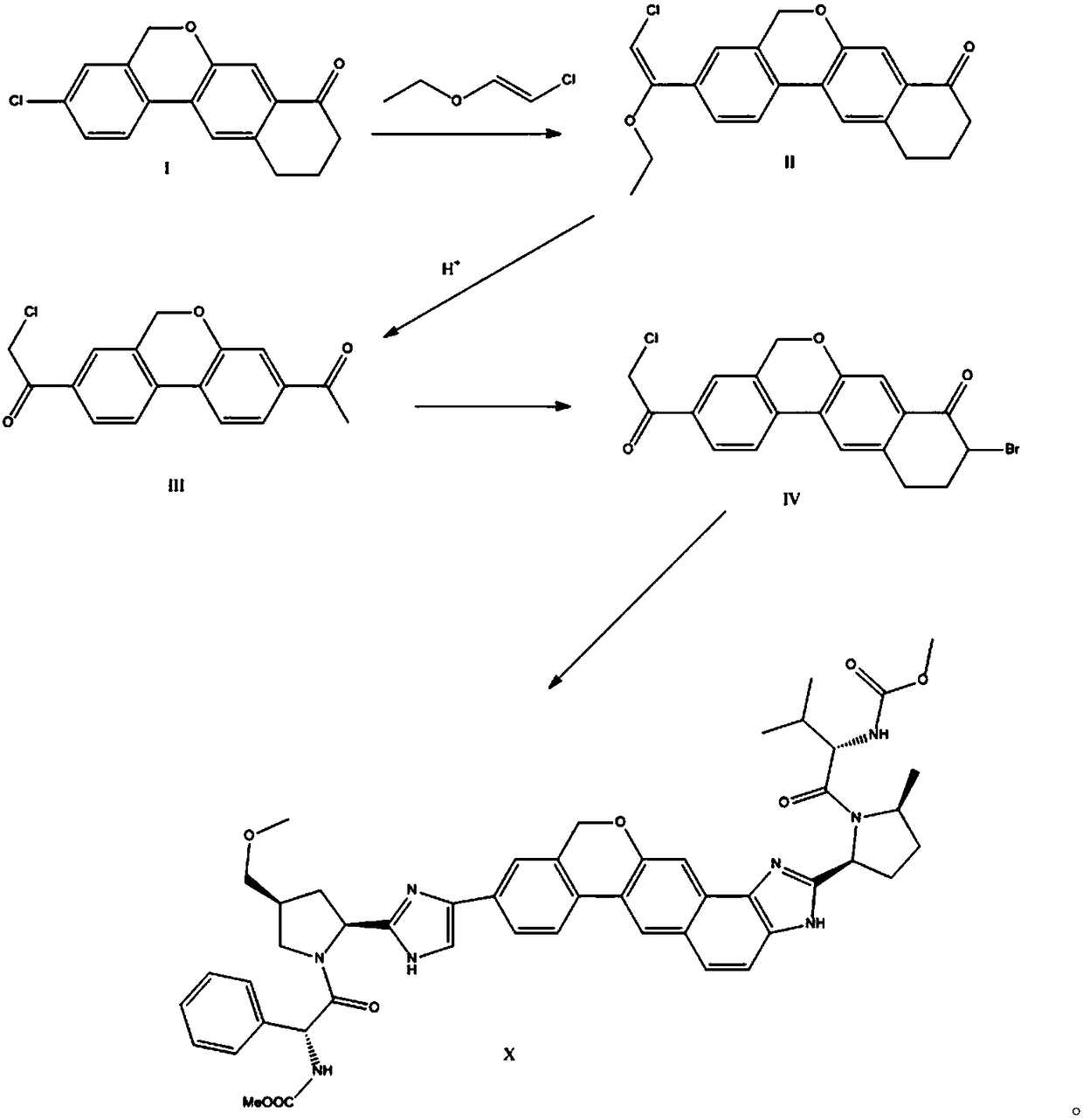
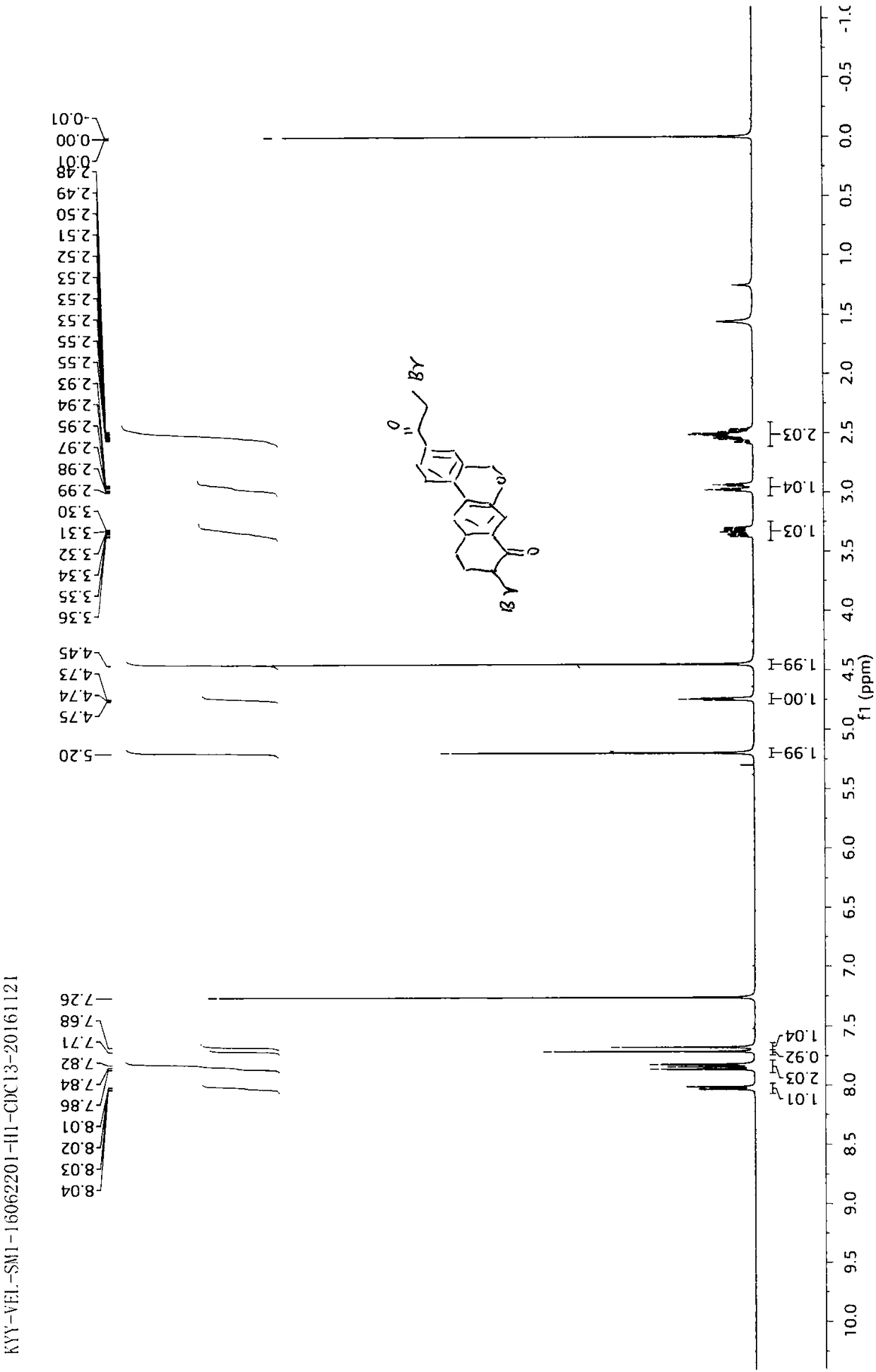
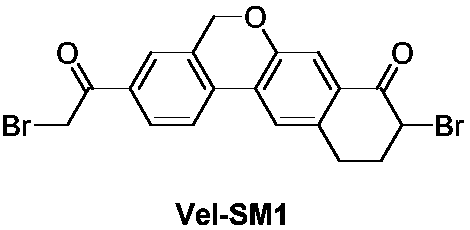
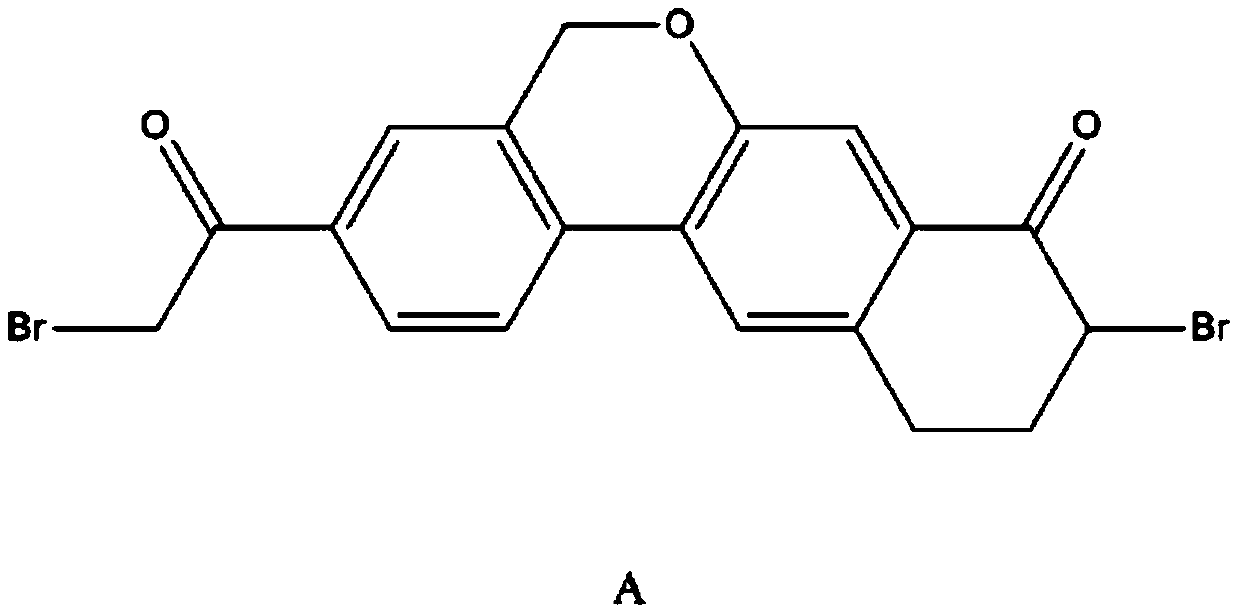
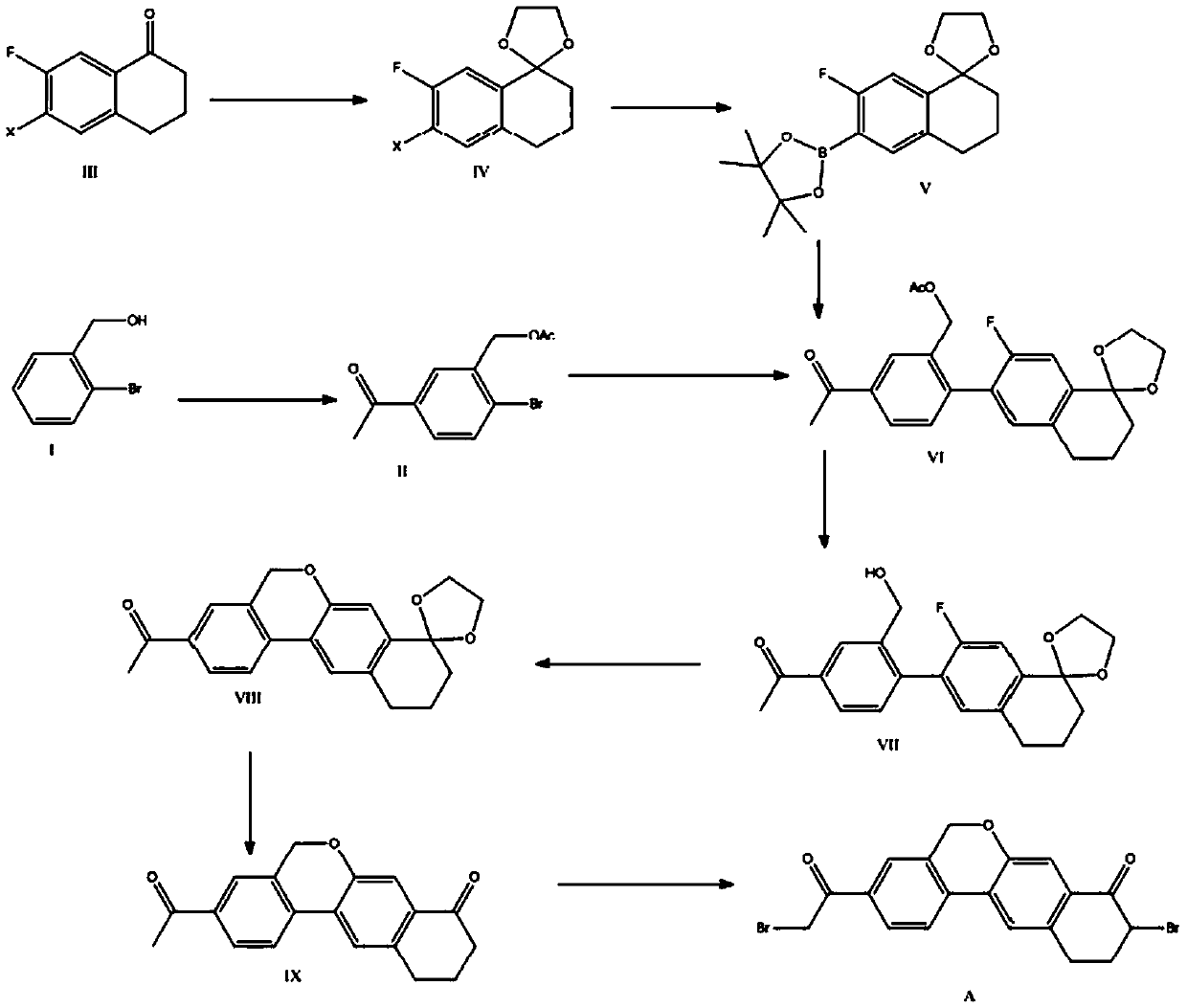
The invention provides a preparation method of a velpatasvir intermediate. According to the preparation method, ortho-toluidine is taken as an initial raw material, amino acetylation protection, Friedel-Crafts acylation, deamination protection, diazotization, and bromination are adopted so as to obtain 3-bromomethyl-4-bromoacetophenone; and alkylation with 7-hydroxy-1-tetralone, intramolecular coupling, and bromination are adopted so as to obtain 9-bromo-3-(2-bromoacetyl)-10, 11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one. The yield and the purity of the velpatasvir intermediate preparedvia the preparation method are high, cost is low, and large size production is convenient to realize.
Owner:安徽省诚联医药科技有限公司
Synthetic method for Velpatasvir intermediate
ActiveCN105712969AOrganic compound preparationCarboxylic acid esters preparationOrganic chemistryVelpatasvir
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
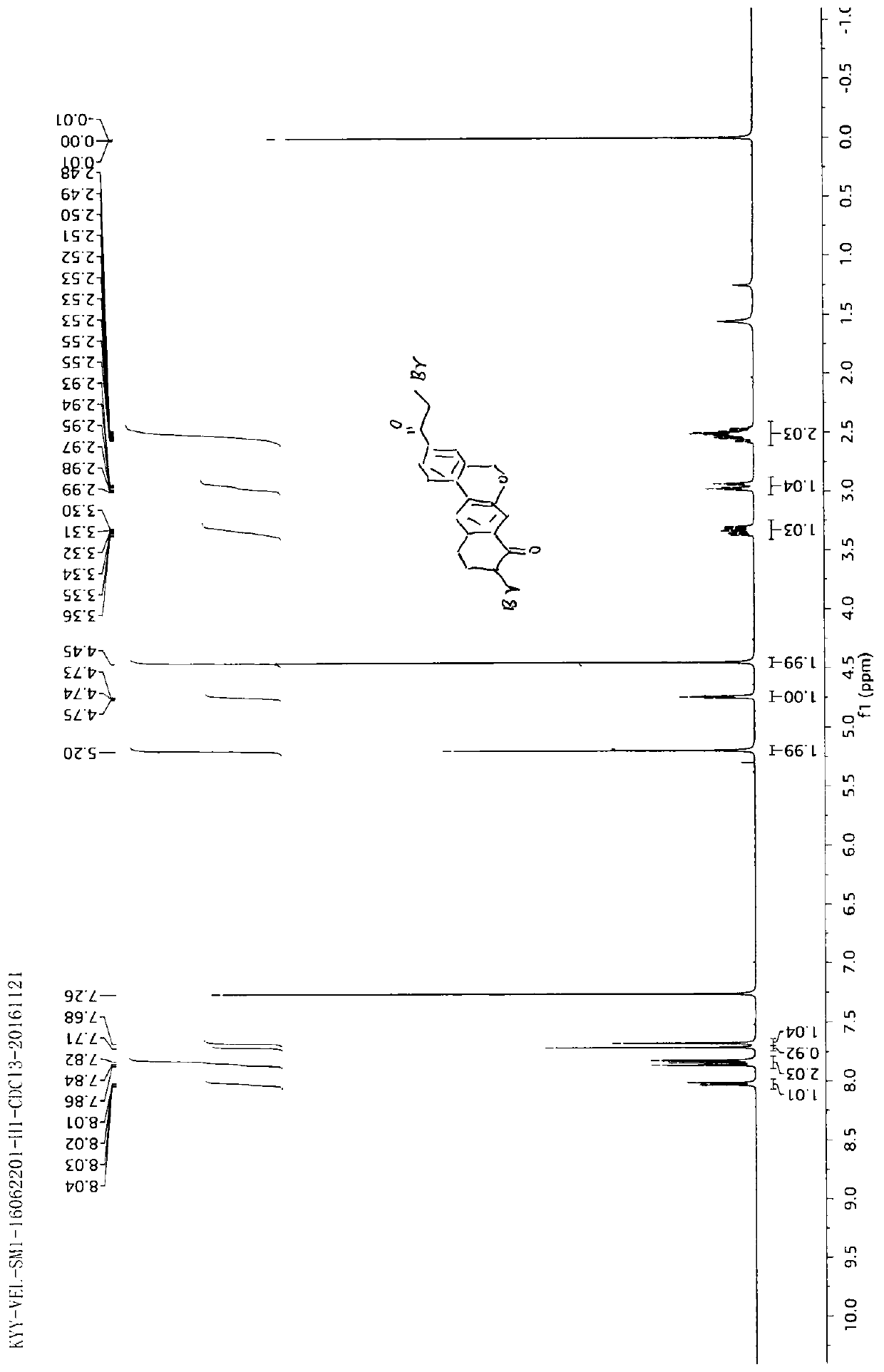
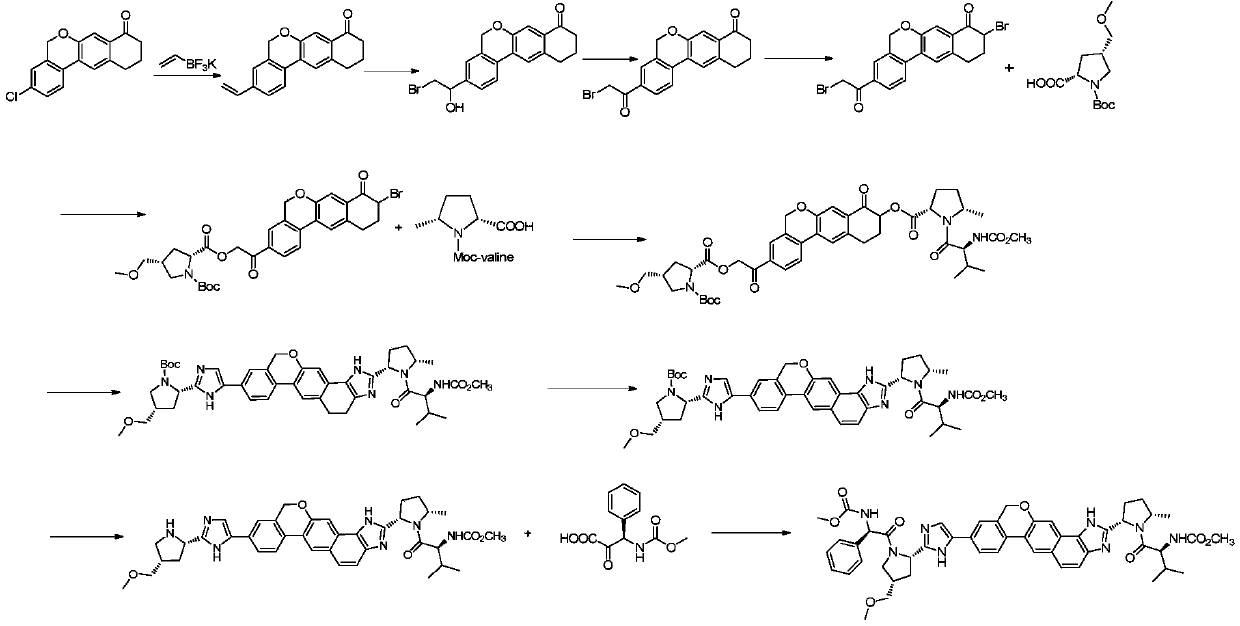
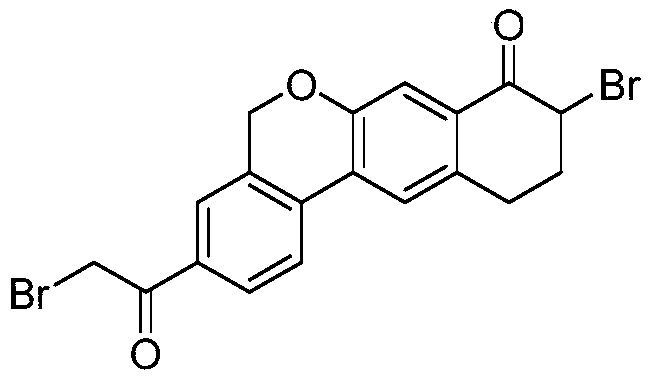
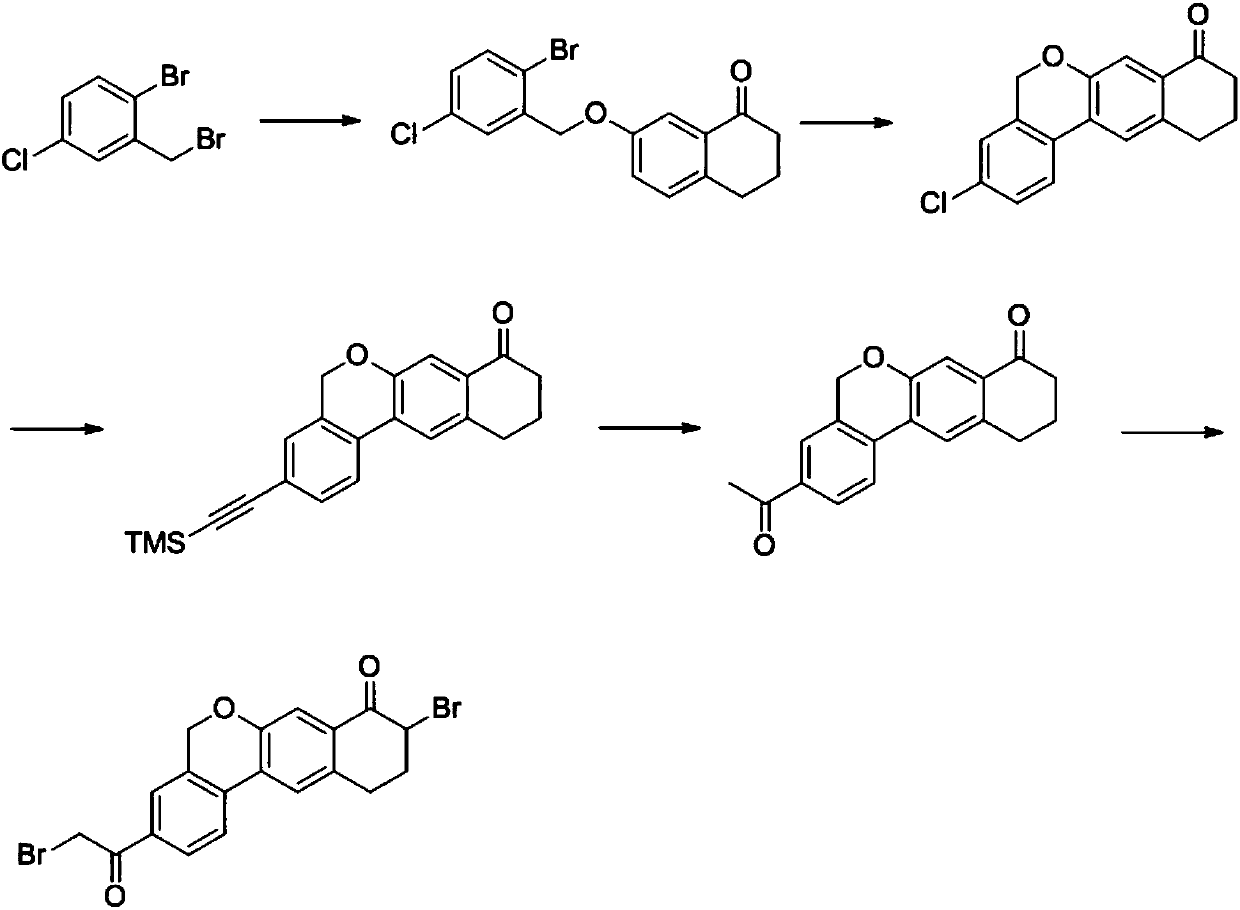
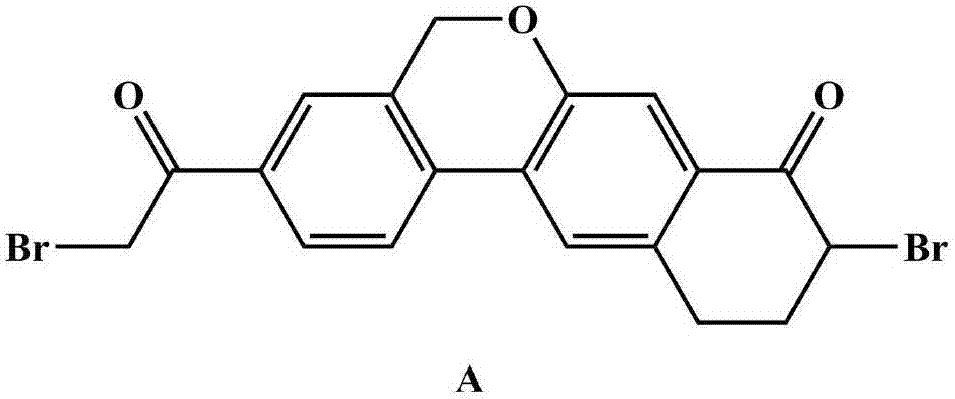
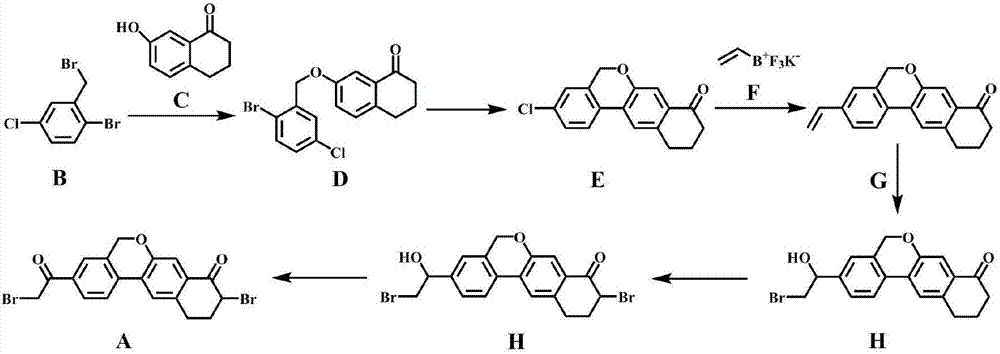
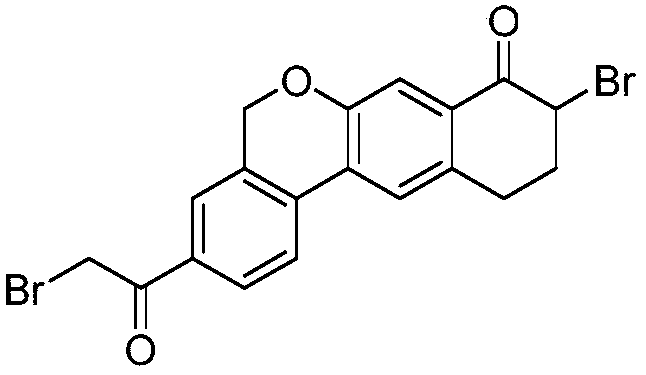
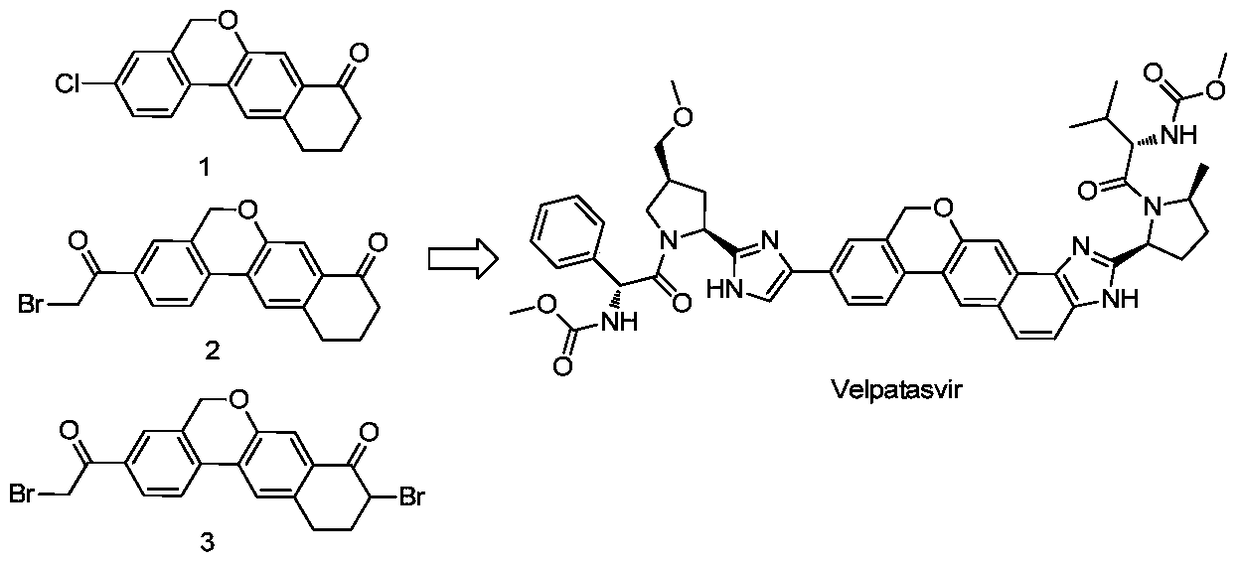
Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone
The invention relates to a novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone. Cheap and available raw materials are utilized; the obtained compound can be applied to synthesis of an anti-hepatitis c medicine velpatasvir; the reaction is shown in the specification.
Owner:SULI PHARMA TECH JIANGYIN
Velpatasvir as well as intermediate and preparation method thereof
ActiveCN107573355AHigh purityMild reaction conditionsOrganic chemistryBulk chemical productionOrganic solventActive ingredient
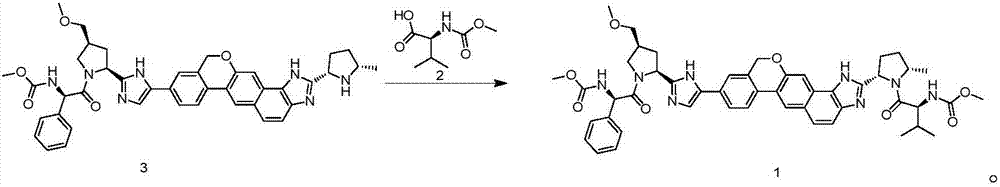
The invention discloses velpatasvir as well as an intermediate and a preparation method thereof. The preparation method of velpatasvir 1 provided by the invention comprises the following steps that inan organic solvent, under the condition of existence of alkali, catalysts and condensating agents, a compound 3 and MOC-L-valine take condensation reaction to obtain velpatasvir 1. The preparation method has the advantages that the reaction conditions are mild; the operation is simple and safe; specific purification equipment is not needed; the column chromatography separation operation in the aftertreatment process is avoided; the chiral isomer is easy to control; the yield is high; the chemical and optical purity of the prepared velpatasvir is greater than 99.50 percent; all impurities aresmaller than 0.10 percent; the raw material medicine standard can be reached; the cost is low; the preparation method is suitable for industrial production. The formula is shown in the description.
Owner:上海云晟研新生物科技有限公司
Synthesis method of velpatasvir
The invention provides a synthesis method of velpatasvir. The method comprises the following steps that reaction is performed on a compound of a structure shown as a formula (II) and a compound of a structure shown as a formula (III) to obtain a compound of a structure shown as a formula (IV); then, the compound of the structure shown as the formula (IV) is converted into the compound shown as the formula (I); the compound of the structure shown as the formula (II) and the compound of the structure shown as the formula (III) are directly subjected to carbon-hydrogen activation coupling; the generation of feature impurities in the synthesis step can be effectively avoided; meanwhile, a final product can be purified through continuous salifying crystallization; the post treatment process is simplified to a great degree; in addition, the purity of the obtained velpatasvir is greater than 99.0 percent; the maximum individual impurities are less than 0.1 percent.
Owner:ANHUI TWISUN HI TECH PHARM CO LTD
Preparation method of velpatasvir intermediate
InactiveCN107501229AStarting materials are cheap and readily availableMild reaction conditionsOrganic chemistryBromineCombinatorial chemistry
The invention discloses a preparation method of velpatasvir intermediate, namely [9-bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-bibenzo(c,g) chromene-8(9h)-one]. The structure of the intermediate corresponds to a formula VI as shown in the description. The method comprises the steps of by taking a compound, namely 4-bromoacetophenone as raw materials, carrying out chloromethylation, condensation, cyclization and bromination, and finally obtaining the velpatasvir intermediate of the formula VI. According to the method, the starting raw materials are cheap and easily available, the reaction conditions are mild, the rout is short, the yield is high and the preparation method is more suitable for industrial production.
Owner:PHARMA SHANGHAI +1
Synthesizing method of velpatasvir
The invention relates to a synthesizing method of velpatasvir. The synthesizing method has the advantages that the novel raw materials and novel intermediates are adopted, the number of reaction stepsis reduced, the usage amount of onium salt is reduced, the defects of longer reaction route, lower total yield rate and unsuitability to industrial production amplification in the synthesizing methodof the prior art are overcome, and the production cost is effectively reduced.
Owner:ZHEJIANG YONGTAI PHARMA
Velpatasvir intermediate as well as preparation method and application thereof
ActiveCN107573380AHigh purityMild reaction conditionsGroup 5/15 element organic compoundsBulk chemical productionVelpatasvirColumn chromatography
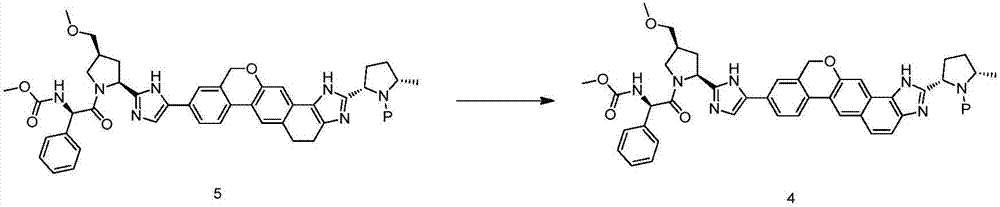
The invention discloses a velpatasvir intermediate as well as a preparation method and application thereof. The preparation method of a velpatasvir intermediate compound 4 provided by the invention comprises the following steps that under the protection of protection gas, in the organic solvent, a compound 5 and an oxidizing agent take oxidation reaction to obtain a compound 4. The velpatasvir isprepared from the intermediate compound 4; the reaction conditions are mild; the operation is simple and safe; specific purification equipment is not needed; the column chromatography separation operation in the aftertreatment process is avoided; the chiral isomer is easy to control; the yield is high; the chemical and optical purity of the velpatasvir prepared from the intermediate compound 4 isgreater than 99.50 percent; all impurities are smaller than 0.10 percent; the raw material medicine standard can be reached; the cost is low; the preparation method is suitable for industrial production. The formula is shown in the description.
Owner:上海云晟研新生物科技有限公司
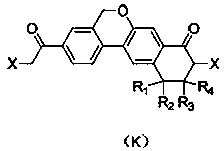
Preparation method of velpatasvir intermediate and analogue thereof
ActiveCN108147972AAvoid PurificationEasy to operateOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryColumn chromatography
The invention discloses a preparation method of a compound shown by formula (E), a velpatasvir intermediate shown by formula (K) and an analogue thereof. Materials adopted in the method disclosed by the invention are low-cost and easily obtained; the technological operation is simple; the intermediate and the product need not be subject to column chromatography separation; and the preparation method is suitable for industrialized mass production.
Owner:XILING LAB CO LTD
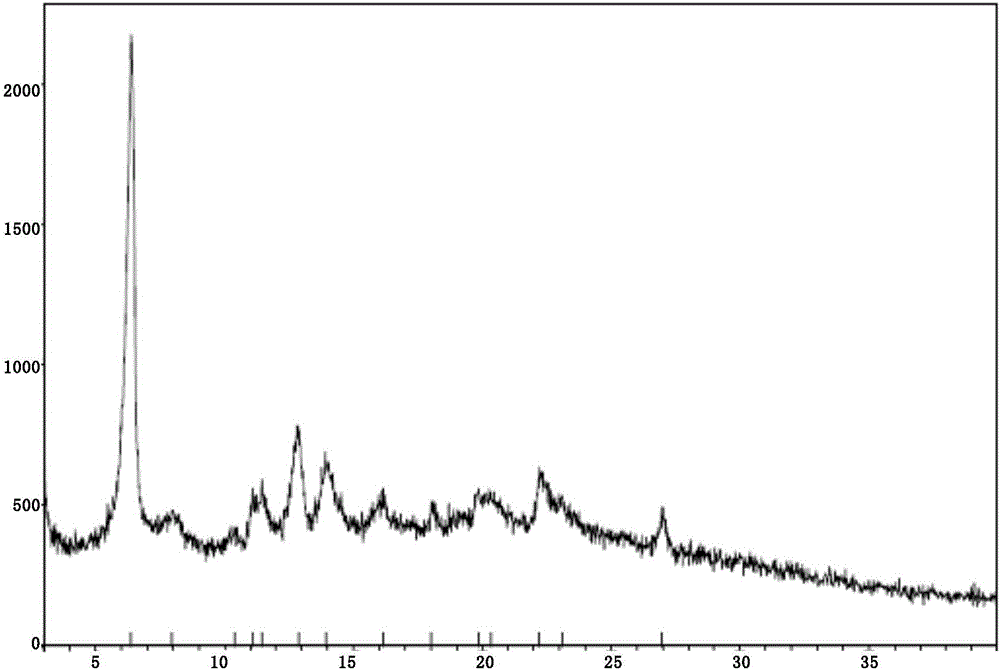
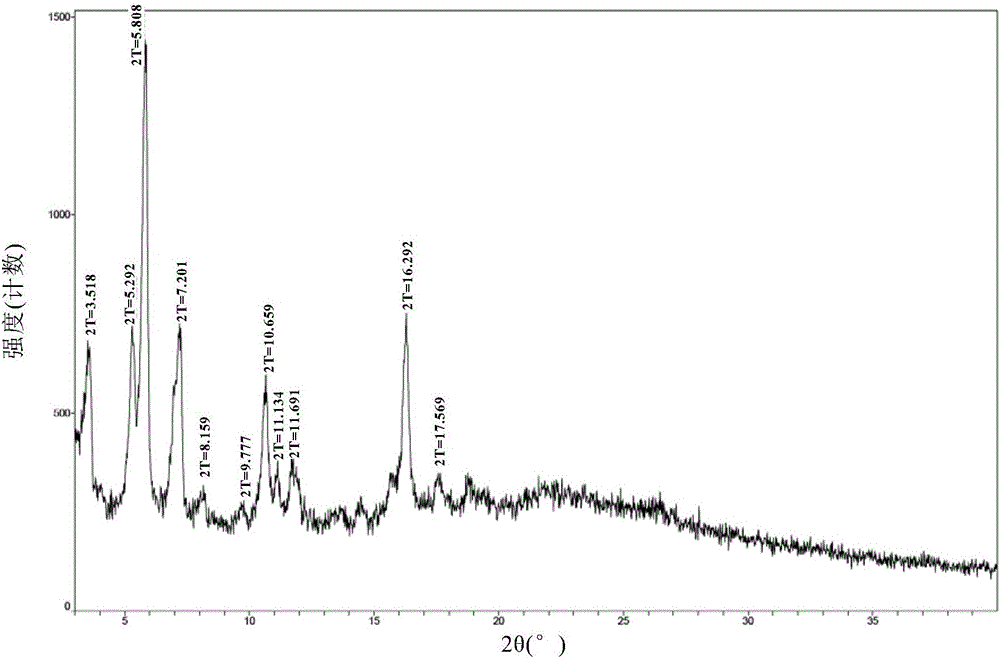
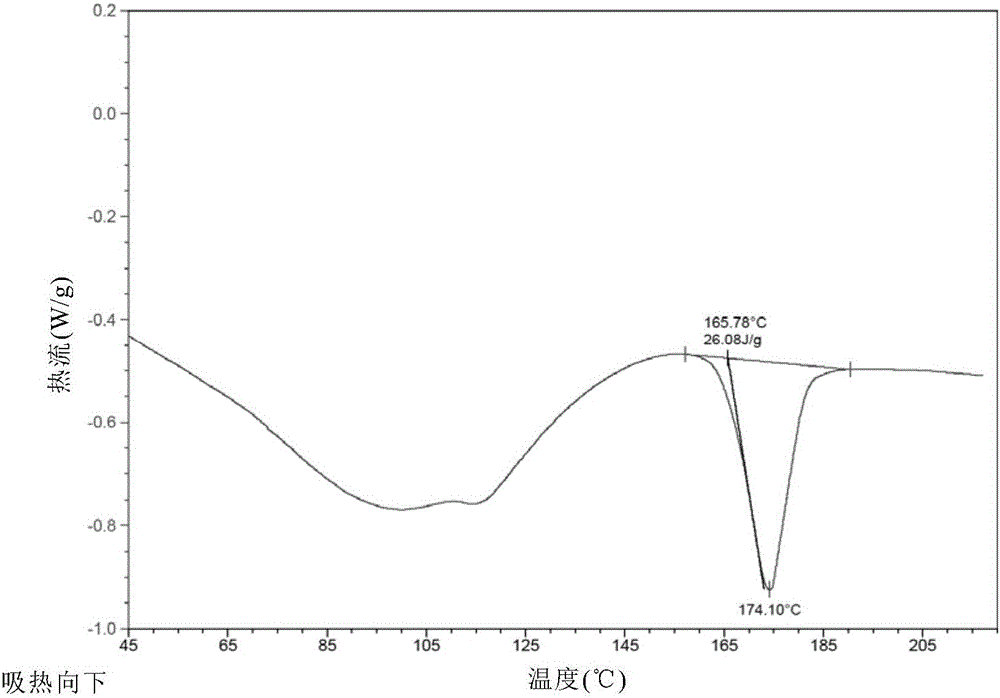
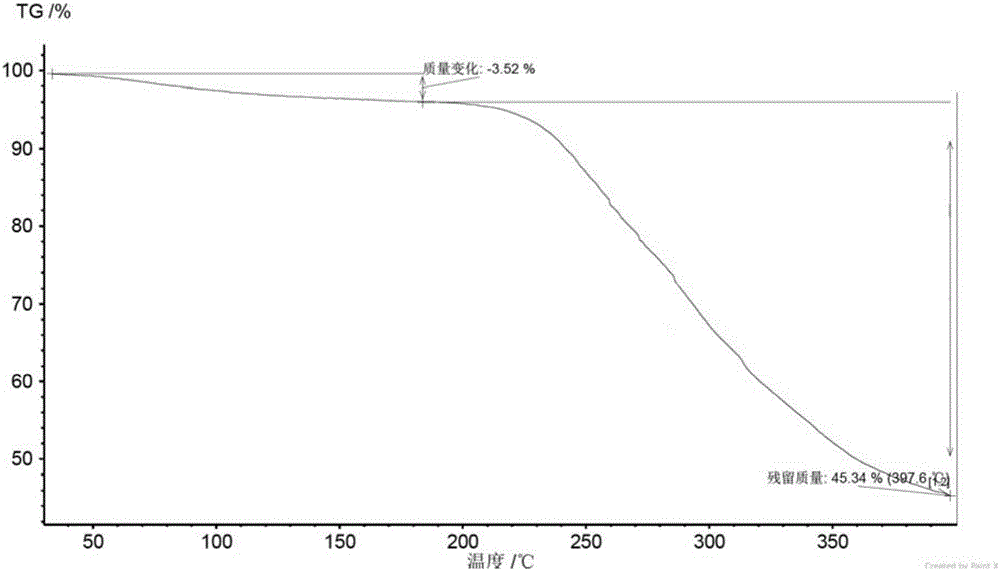
New velpatasvir intermediate crystal form
The invention relates to a new velpatasvir intermediate crystal form. Specifically, the invention discloses a polymorphic substance of the formula I compound and a preparation method thereof. The polymorphic substance has good purifying effect.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
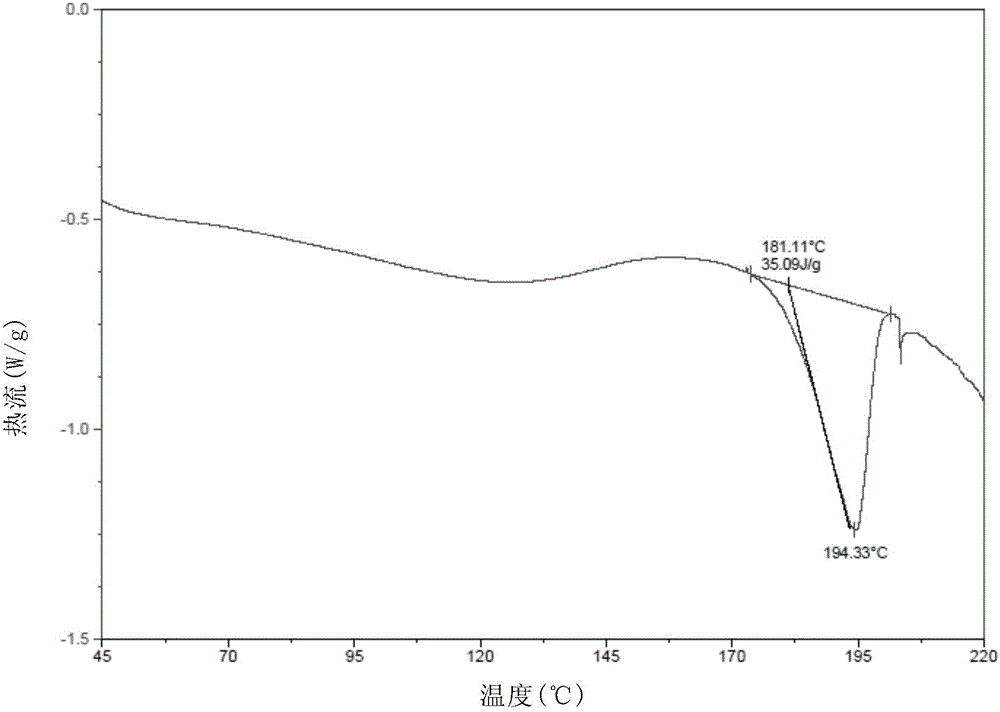
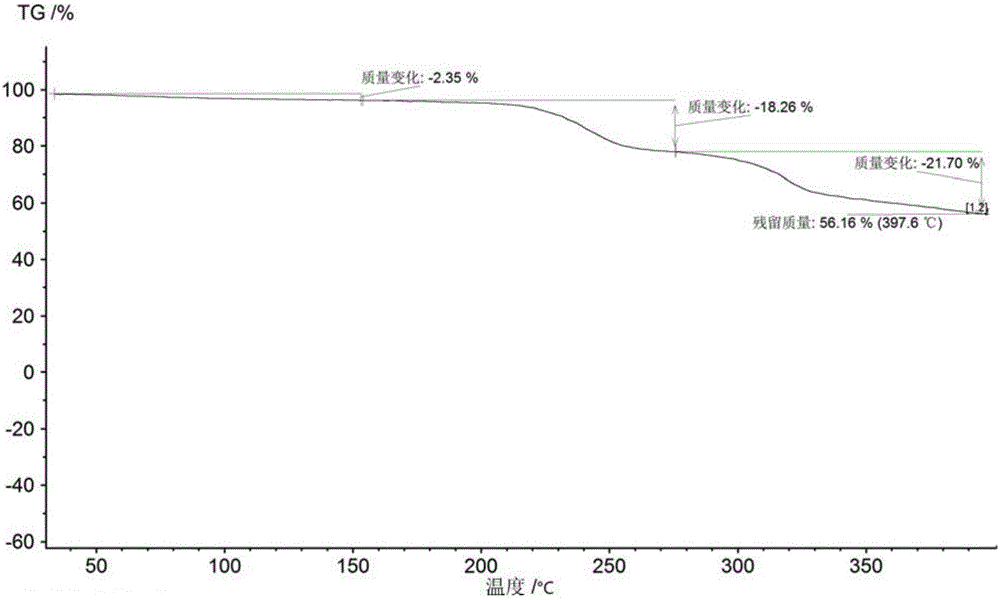
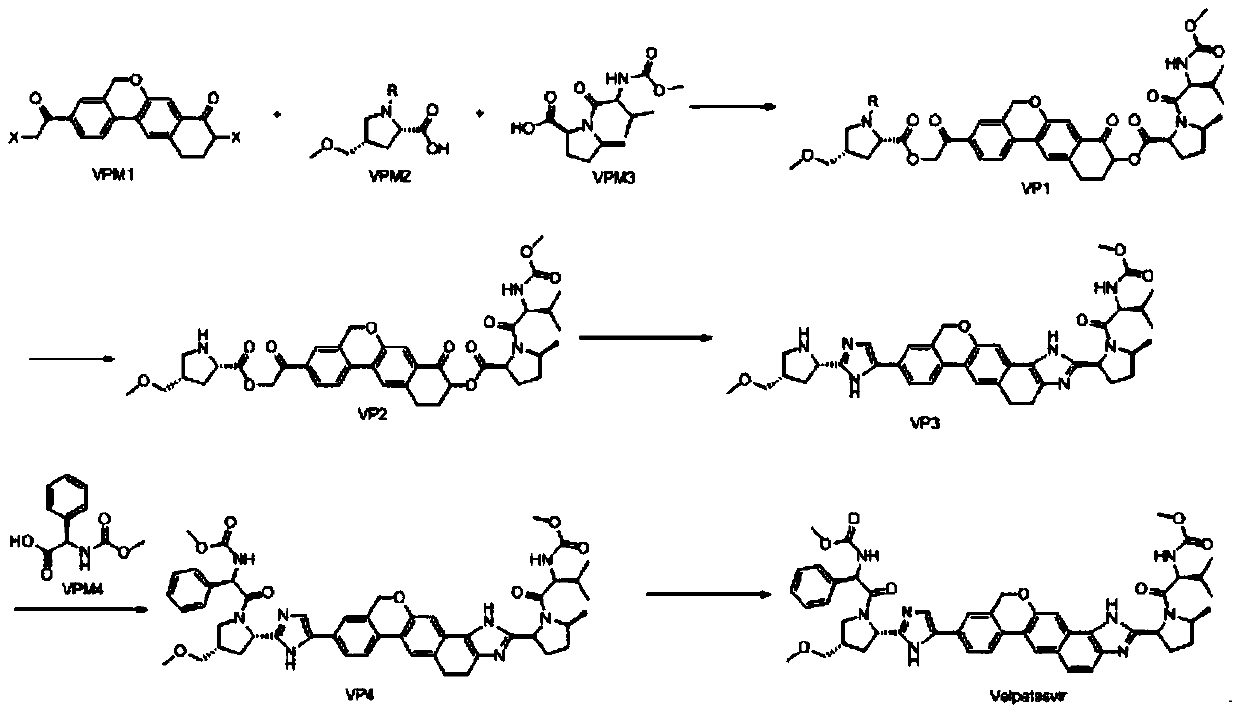
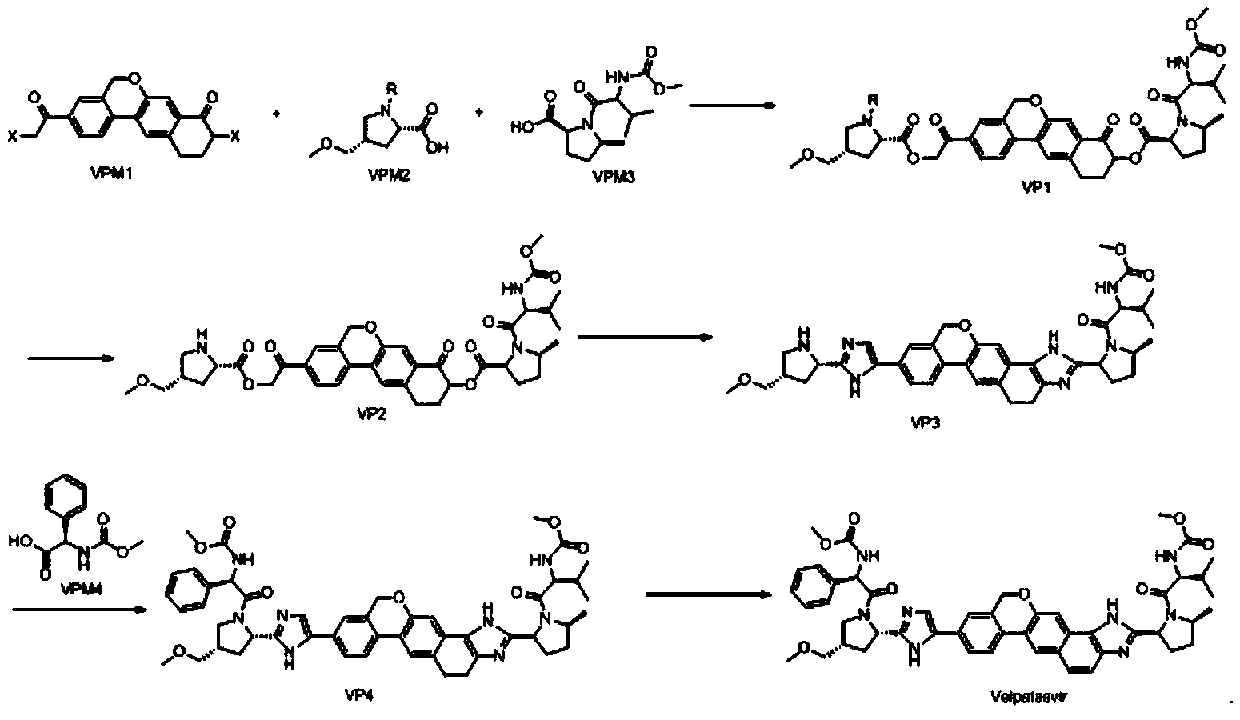
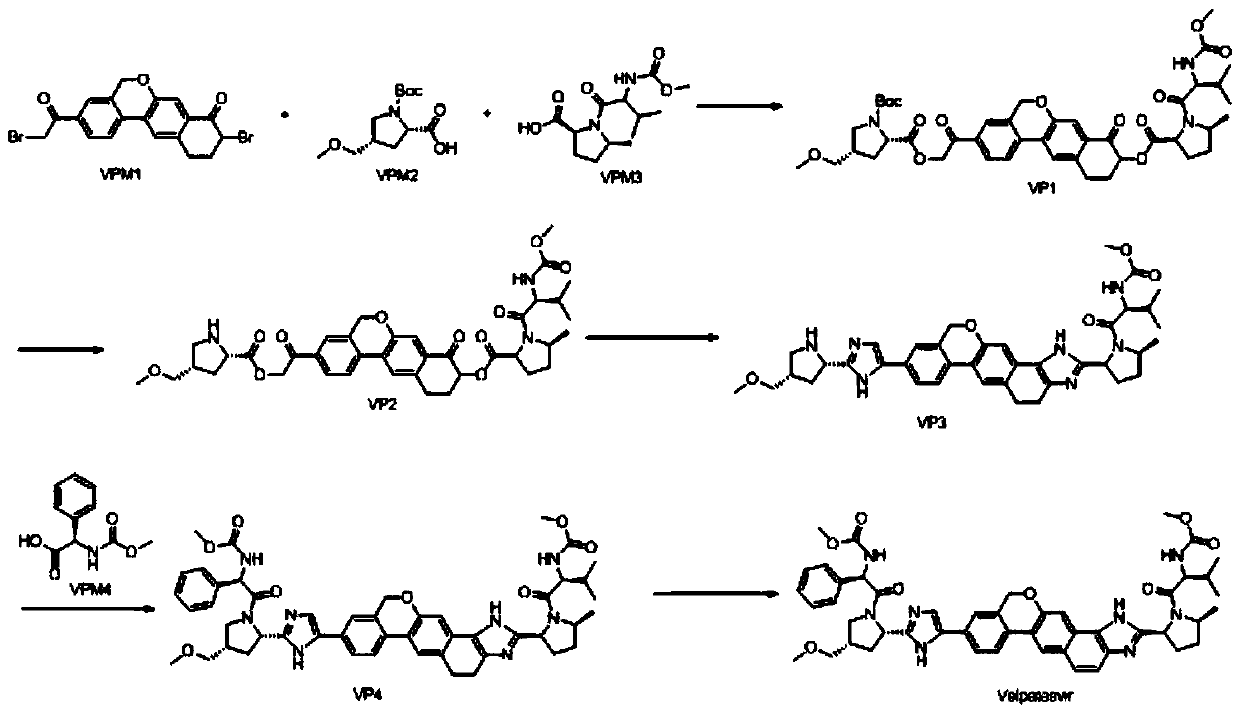
Method for preparing NS5A inhibitor-Velpatasvir
ActiveCN110981879ABeneficial to expand the applicationSimple reaction conditionsOrganic chemistryBulk chemical productionCombinatorial chemistryProtecting group
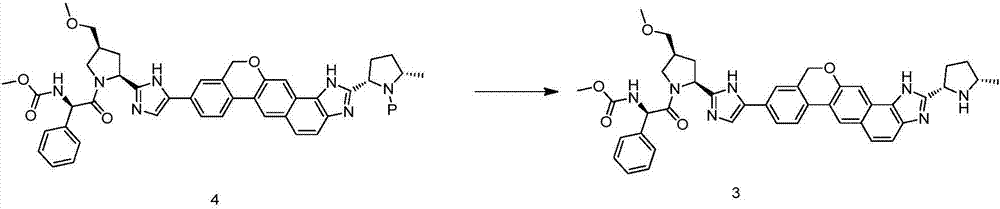
The invention discloses a method for preparing an NS5A inhibitor-Velpatasvir, wherein the method mainly comprises the five steps: 1) carrying out docking reaction on reaction initial raw materials VPM1, VPM2 and VPM3 under the catalytic action of alkali to prepare an intermediate VP1; 2) removing amino protecting groups from the intermediate VP1 by using a protecting group removing reagent to prepare an intermediate VP2; 3) carrying out cyclization on the intermediate VP2 and an amine compound to prepare an intermediate VP3; 4) carrying out a reaction on the intermediate VP3 with VPM4 under the action of a condensing agent to prepare an amide compound intermediate VP4; and 5) carrying out oxidation reaction on the intermediate VP4 under the action of an oxidizing agent to prepare Velpatasvir. The preparation process of Velpatasvir has the characteristics of simplified route, simple reaction conditions, enhanced operability and higher product yield, and is more suitable for large-scaleindustrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Synthesis method of Velpatasvir intermediate A
InactiveCN107311852AMild reaction conditionsSimple stepsPreparation from carboxylic acid halidesOrganic compound preparationSynthesis methodsCombinatorial chemistry
The embodiment of the invention discloses a synthesis method of a Velpatasvir intermediate A, and further provides three novel compounds for synthesis of the intermediate A: a compound I, a compound I1 and a compound I2. The method for the synthesis of the Velpatasvir intermediate A has the characteristics of low cost, environment friendliness and suitability for industrialized production.
Owner:SHANGHAI TWISUN BIO PHARM
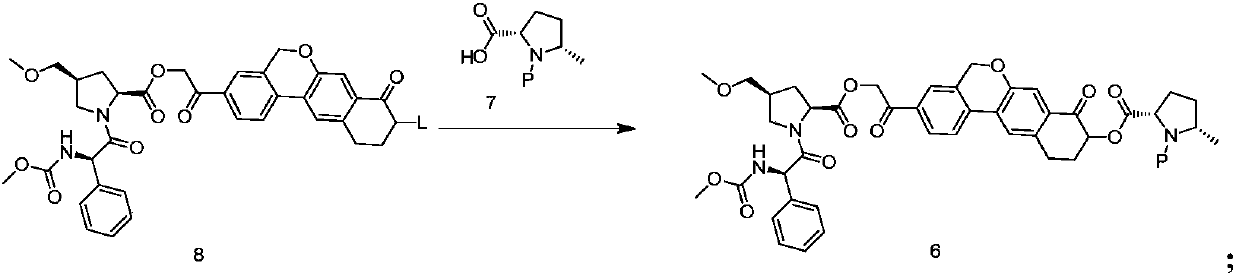
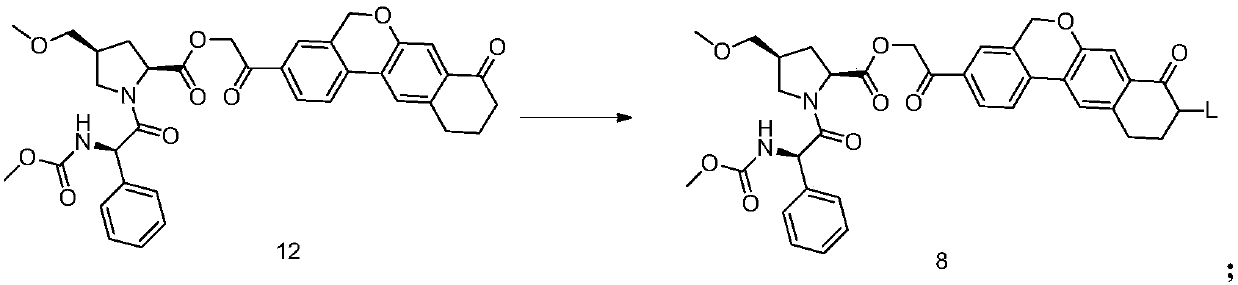
Velpatasvir intermediate, preparation method thereof and preparation of Velpatasvir
ActiveCN107674064AHigh purityMild reaction conditionsPeptide preparation methodsBulk chemical productionOrganic solventShielding gas
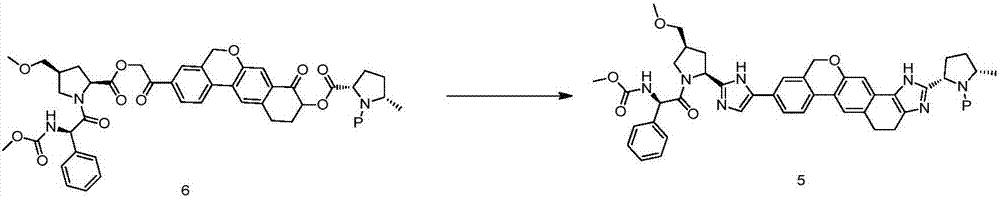
The invention discloses a Velpatasvir intermediate, a preparation method thereof and a preparation of Velpatasvir. The invention provides a preparation method of a Velpatasvir intermediate compound 6,which comprises the the step: performing a reaction of nucleophilic substitution on a compound 8 and a compound 7 in an organic solvent under a condition that alkali exists under the protection of protective gas, so as to obtain the compound 6. The preparation method provided by the invention is mild in reaction conditions and simple and safe in operation and does not need special purification equipment, column chromatography isolation operation in an aftertreatment process is avoided, and a chiral isomer is controlled easily; and the yield is high, the chemical and optical purity of Velpatasvir 1 prepared from the intermediate compound 6 are greater than 99.50%, and all impurities are less than 0.10%, so that the Velpatasvir 1 can reach the crude drug standard, is low in cost and is suitable for industrial production.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
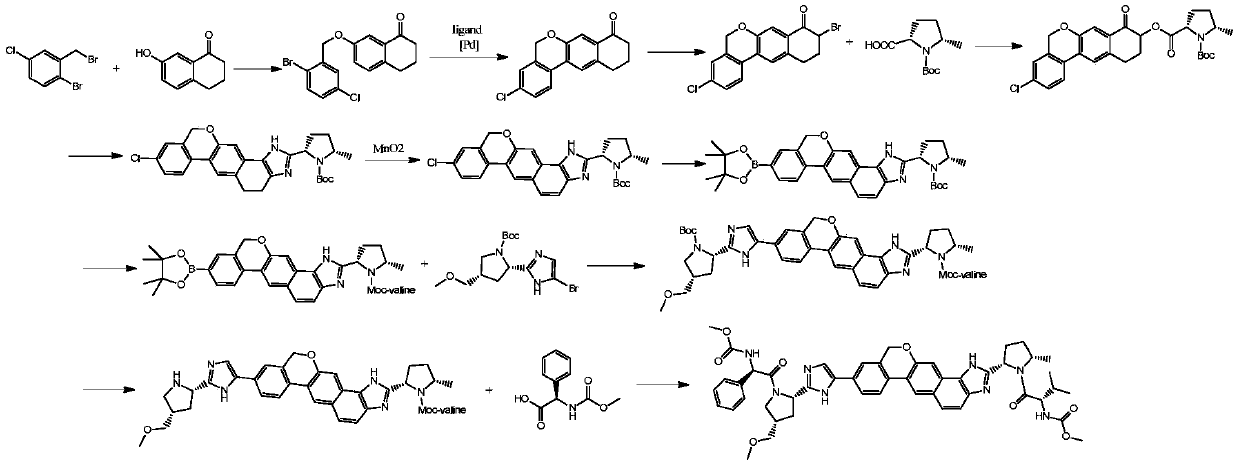
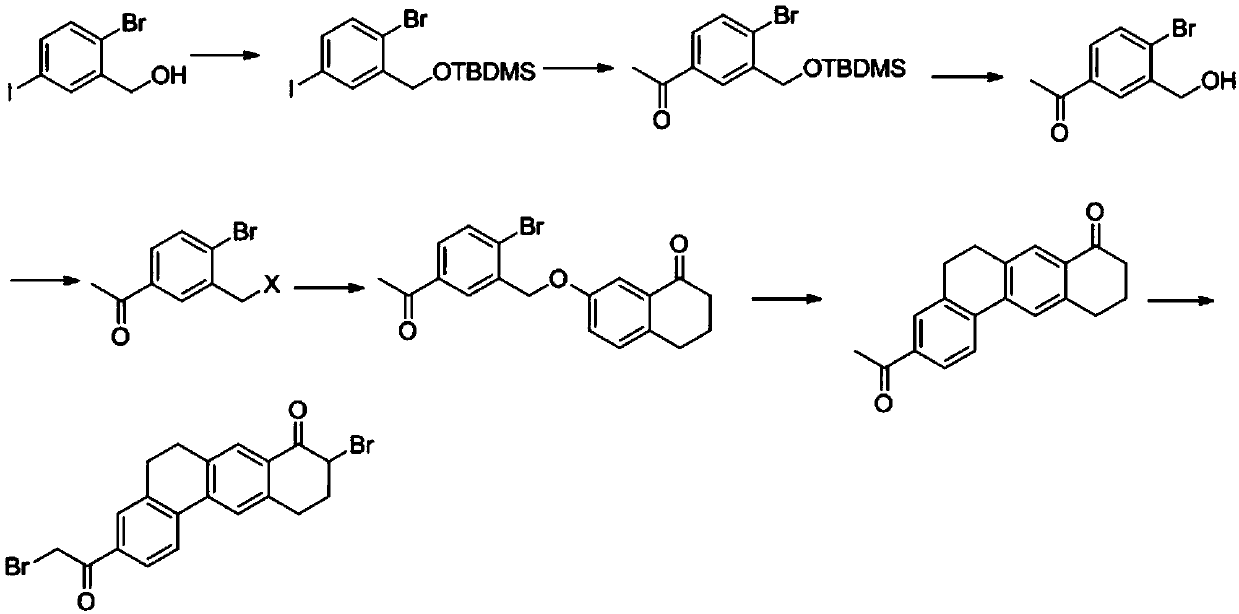
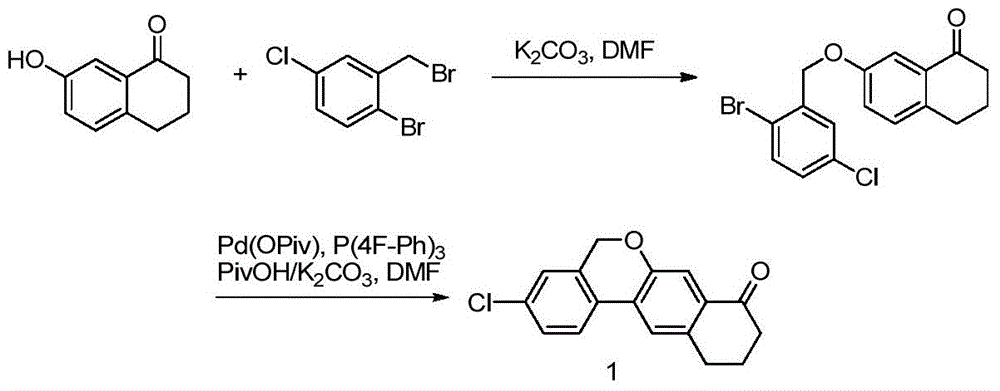
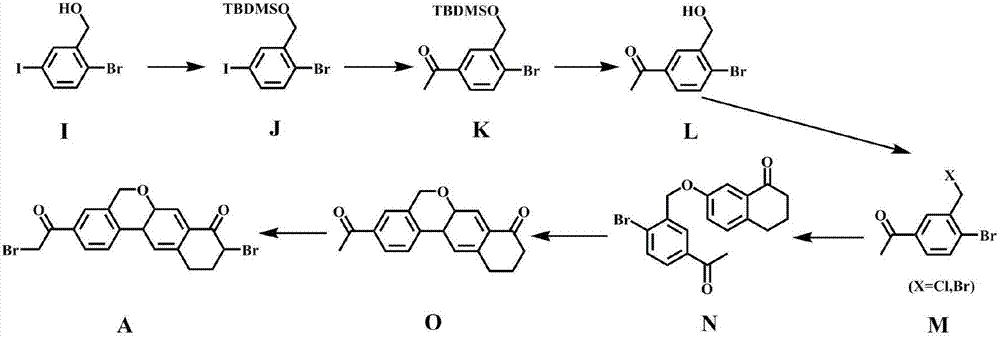
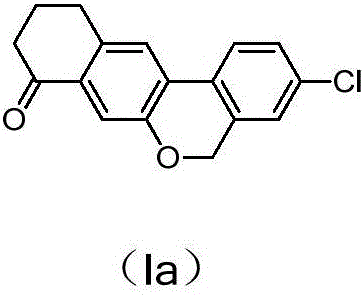
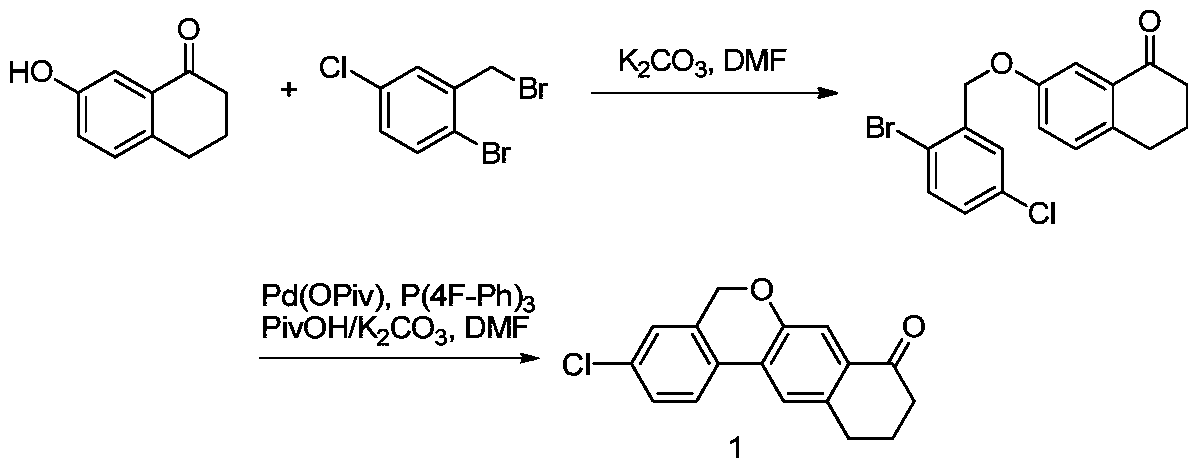
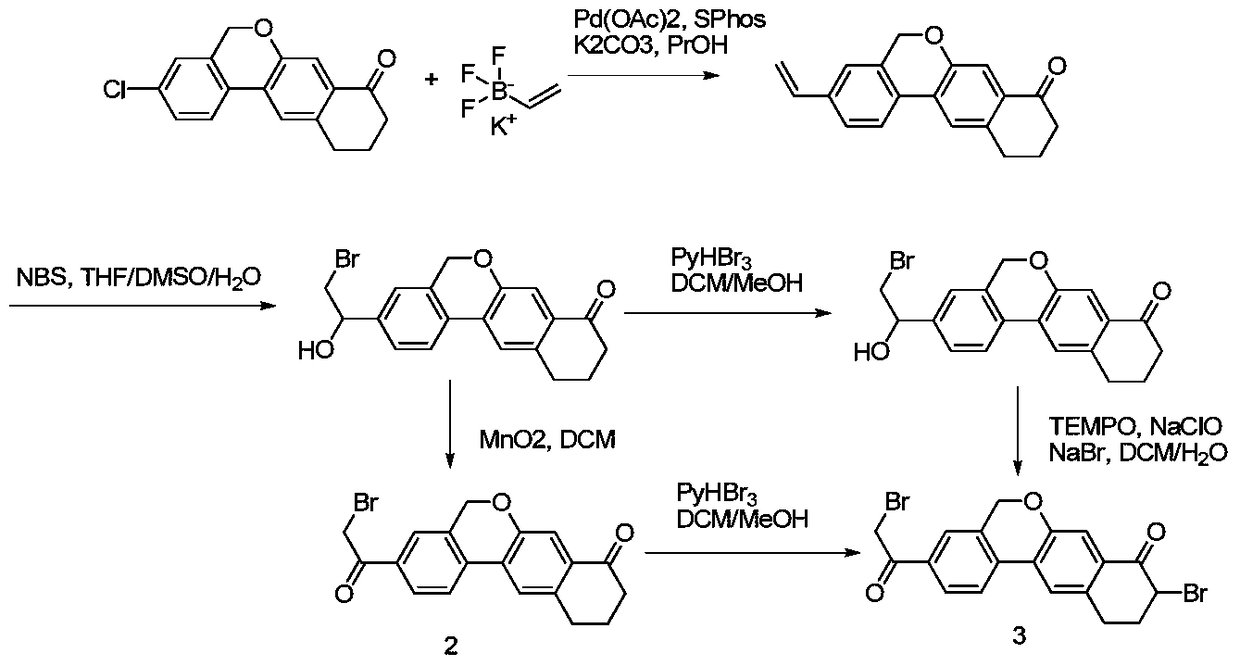
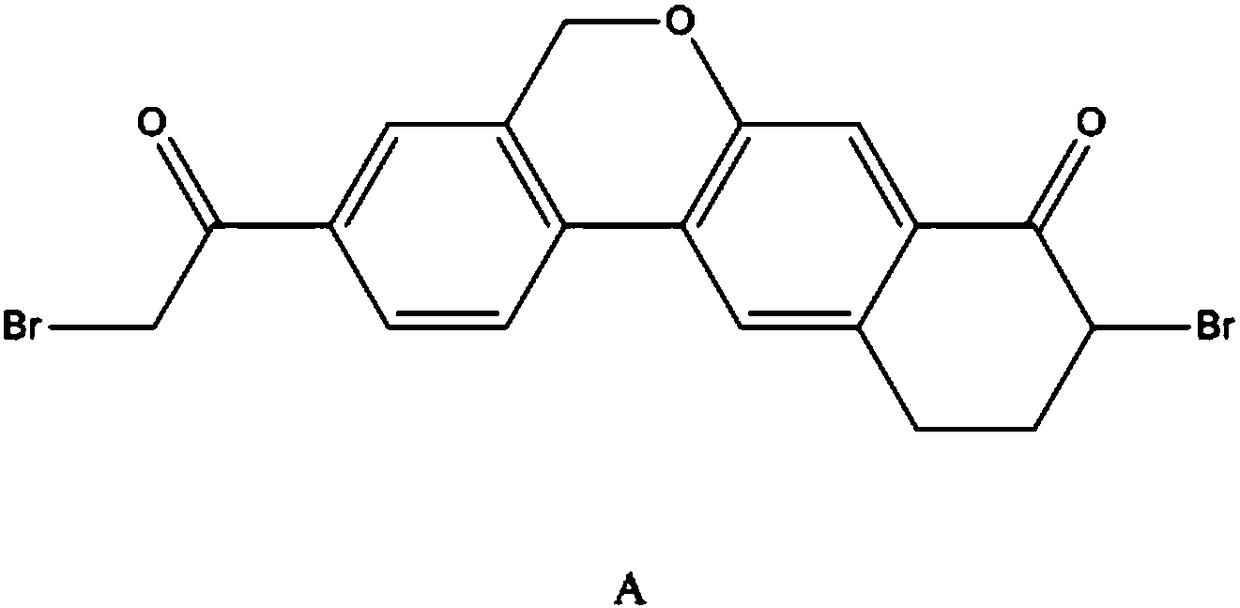
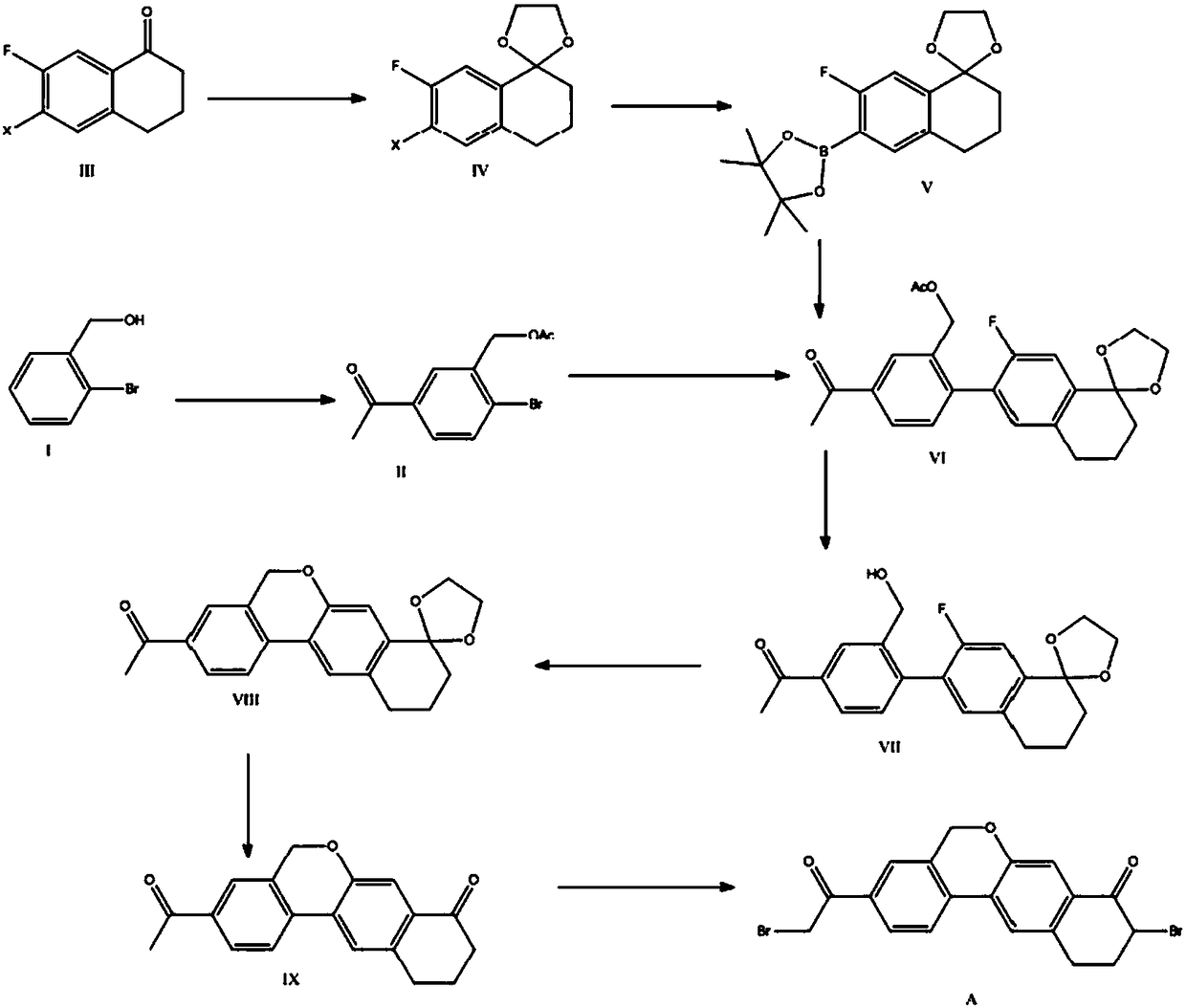
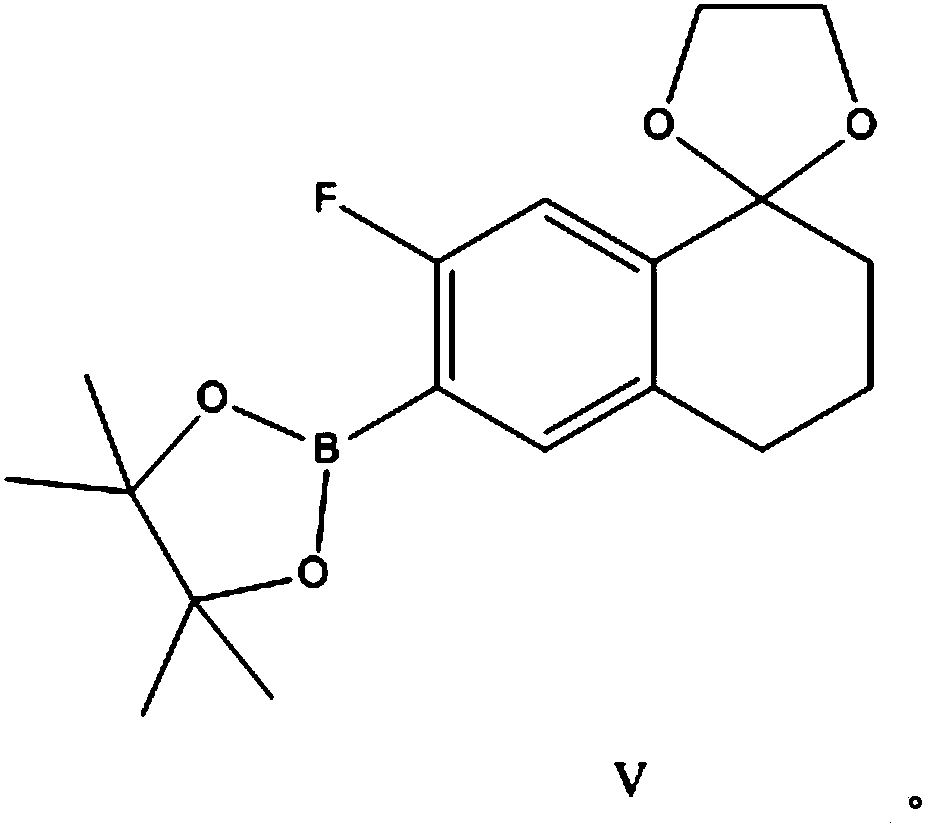
Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative
ActiveCN106916134AThe reaction steps are easy to controlStable industrial production preparationOrganic chemistryLithiumOrganic acid
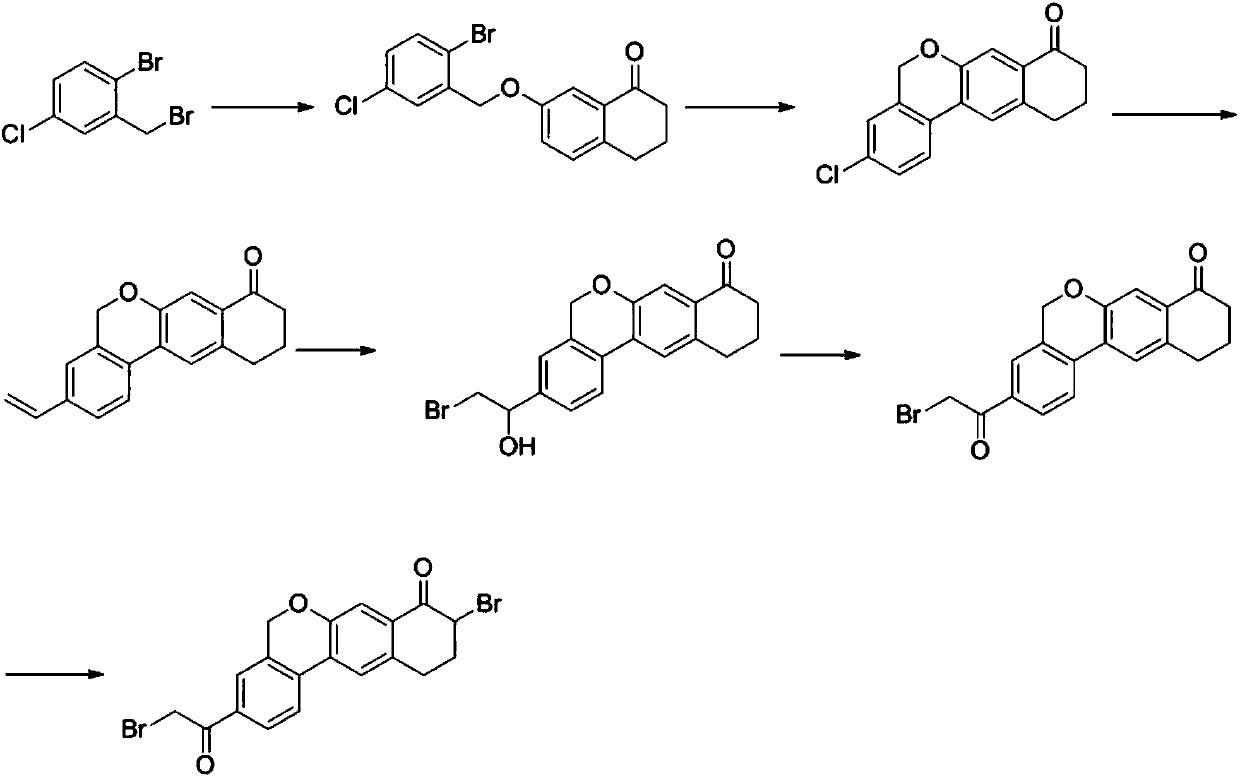
The invention discloses a preparation method of an anti-hepatitis C medicine Velpatasvir intermediate 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative which is 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one (V). The preparation method concretely comprises the following steps: carrying out coupling cyclization on a raw material 7-((2-bromo-5-chlorophenyl)oxo)-3,4-dihydronaphthalen-1(2H)-one under the action of a ligand and an alkali to synthesize 3-chloro-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one, substituting chlorine by using sodium cyanide to form cyanide, prearing an organic acid from the cyanide under the action of an alkali, processing the organic acid by a lithium methide reagent to obtain 3-acetyl-10,11-dihydro-5H-benzo[d]naphtho[2,3-b] ]pyran-8(9H)-one, and carrying out a two-step bromination reaction to obtain the anti-hepatitis C medicine Velpatasvir intermediate. The method provides a brand new synthesis route of the anti-hepatitis C medicine Velpatasvir intermediate, and has the advantages of easily controlled reaction steps, and realization of stable industrial production preparation.
Owner:苏州楚凯药业有限公司
Method for re-crystallizing key intermediate of hepatitis C virus drug velpatasvir
The invention provides a method for re-crystallizing a key intermediate of a hepatitis C virus drug velpatasvir. Highly pure 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8(9H)-one is obtained, and parts of organic solvents can be recovered, so the method is economical and environmentally friendly. The method concretely includes the following steps: dissolving crude 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8(9H)-one in a mixed solvent containing a high-solubility organic solvent A and a low-solubility organic solvent B under a 35-70 DEG Cheating condition, precipitating crystals by a gradient cooling technology, centrifuging the obtained system, carrying out vacuum drying on the obtained wet solid at 30-70 DEG C, and performing normalpressure distillation on the obtained centrifugal mother liquor to recover the solvent B.
Owner:SULI PHARMA TECH JIANGYIN
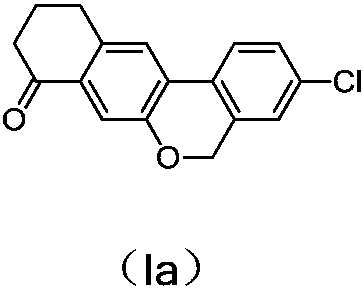
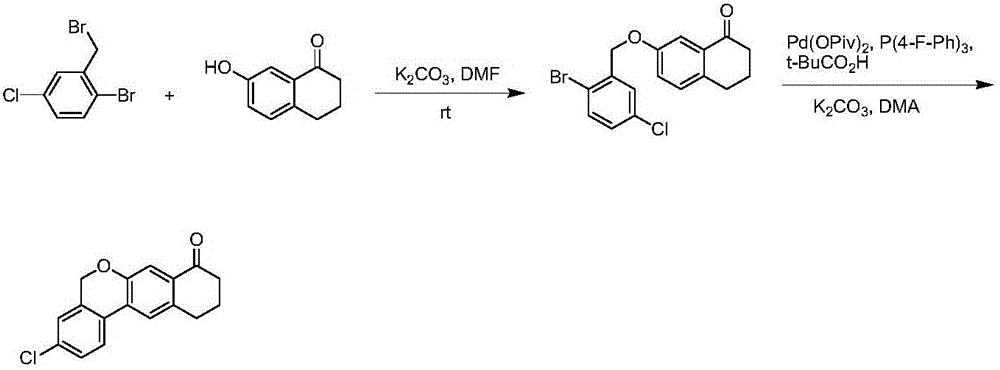
Preparation method of benzochromene derivative
ActiveCN105801553AOrganic compound preparationHydroxy compound preparationBenzeneMedicinal chemistry
The invention discloses a preparation method of a benzochromene derivative shown as a formula (I). The benzochromene derivative can be taken as a synthesis intermediate of a drug such as a synthesis intermediate of Velpatasvir. Cheap and available 2-fluoro halogenated benzene is taken as a starting material, a brand-new synthetic route for preparing the benzochromene derivative is provided, the total yield of the whole reaction route is high, and the method is suitable for large-scale industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
New crystal form of Velpatasvir and preparation method of new crystal form
ActiveCN106432253AHigh crystallinityImprove stabilityOrganic chemistry methodsCombinatorial chemistryVelpatasvir
The invention discloses a new crystal form of Velpatasvir and a preparation method of the new crystal form. The structure of a compound with a formula I is shown as follows; a crystal form A of the compound with the formula I, which is prepared by the preparation method disclosed by the invention, has a good purification effect and physiochemical properties. (The formula I is shown in the description.).
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
A kind of synthetic method of velpatasvir intermediate a
ActiveCN108358881BMild reaction conditionsSimple stepsGroup 3/13 element organic compoundsBiochemical engineeringChemical compound
The invention relates to the technical field of medicine, in particular to a synthesis method of a velpatasvir intermediate A. Proper reactants are selected for synthesis of the velpatasvir intermediate A, a linear synthesis mode is changed into a convergence synthesis mode, and the method is mild in reaction condition, simple in step, high in synthesis efficiency, environmentally friendly and beneficial to industrial production and has good application prospects and market potentials. Besides, the invention also provides an intermediate compound for synthesizing the velpatasvir intermediate A.
Owner:ZHEJIANG YONGTAI PHARMA
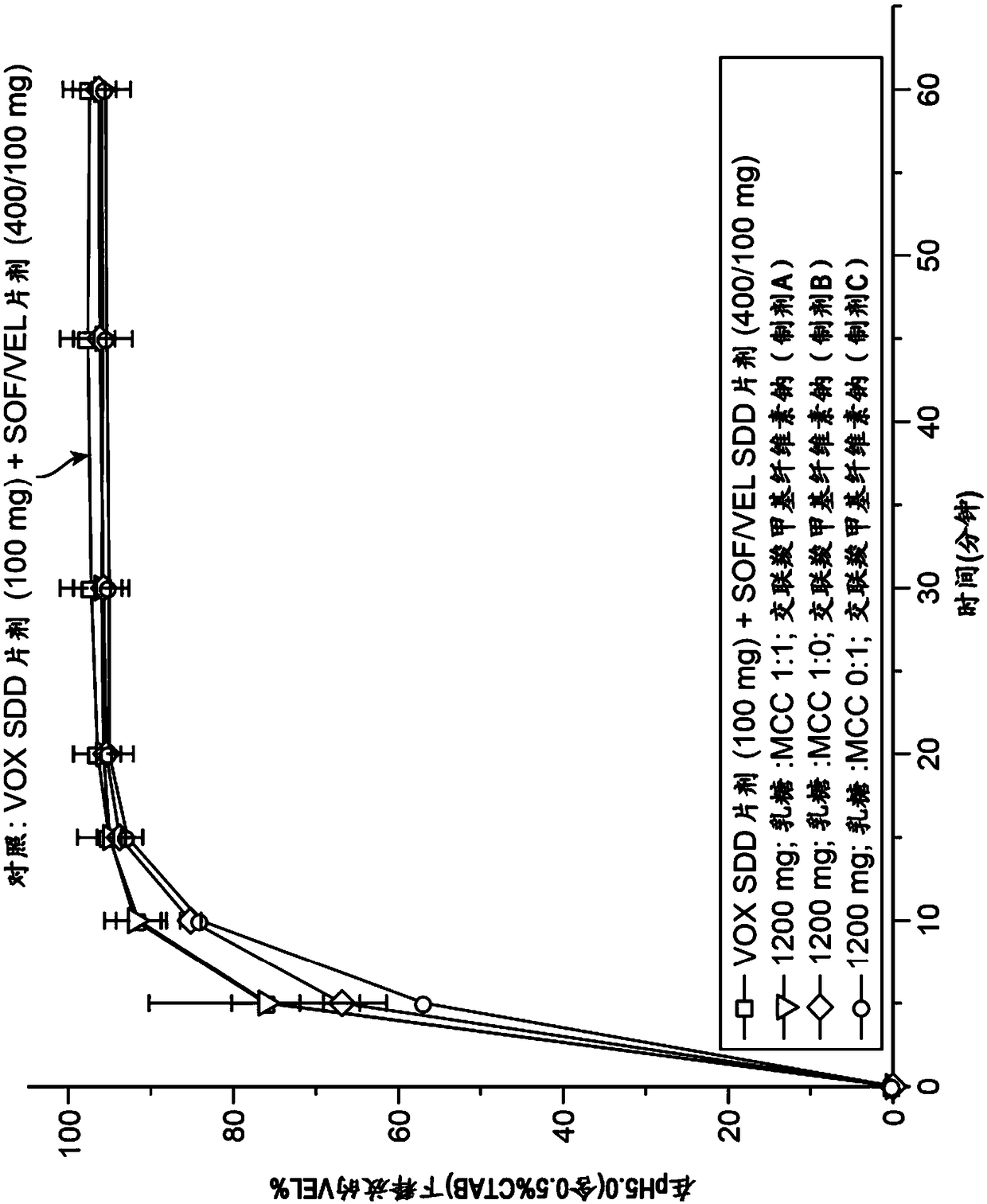
Combination formulation of three antiviral compounds
Disclosed are pharmaceutical compositions comprising three antiviral compounds. In particular, the pharmaceutical compositions comprise an effective amount of velpatasvir, an effective amount of sofosbuvir, and an effective amount of voxilaprevir. Also disclosed are methods of use for the pharmaceutical composition.
Owner:GILEAD PHARMASSET LLC
The synthetic method of velpatasvir intermediate
ActiveCN105712969BOrganic compound preparationCarboxylic acid esters preparationCombinatorial chemistryOrganic chemistry
The invention provides two synthetic methods for a Velpatasvir key intermediate 3 and further provides a new compound I and a new compound I3 for preparing the intermediate 3. Please see the structures of the three compounds in description.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Synthesis method of velpatasvir intermediate A
ActiveCN108358881AMild reaction conditionsSimple stepsGroup 3/13 element organic compoundsSynthesis methodsCombinatorial chemistry
The invention relates to the technical field of medicine, in particular to a synthesis method of a velpatasvir intermediate A. Proper reactants are selected for synthesis of the velpatasvir intermediate A, a linear synthesis mode is changed into a convergence synthesis mode, and the method is mild in reaction condition, simple in step, high in synthesis efficiency, environmentally friendly and beneficial to industrial production and has good application prospects and market potentials. Besides, the invention also provides an intermediate compound for synthesizing the velpatasvir intermediate A.
Owner:ZHEJIANG YONGTAI PHARMA
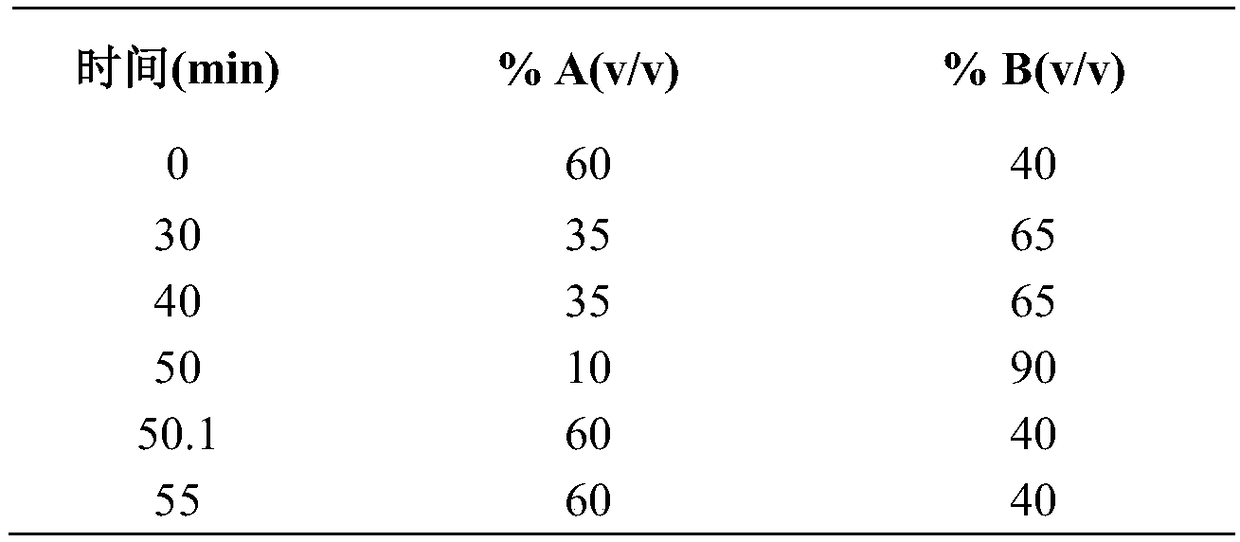
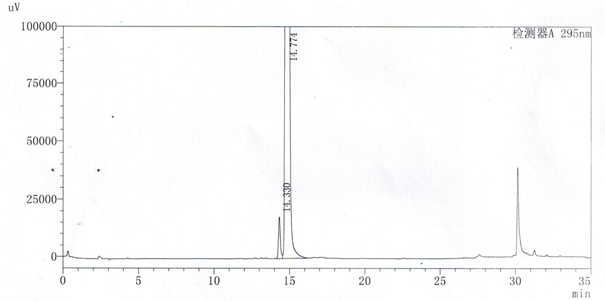
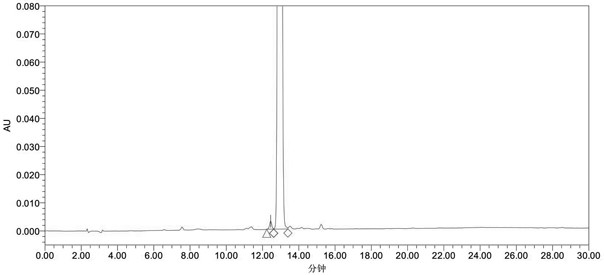
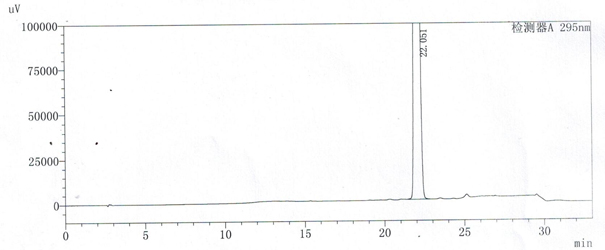
A method for detecting related substances of velpatasvir
ActiveCN107655986BEfficient separationEasy to separateComponent separationO-Phosphoric AcidAqueous solution
The invention relates to a method for detecting velpatasvir related substances. Gradient elution can be carried out by taking a phosphoric acid aqueous solution and acetonitrile as mobile phases, andvelpatasvir can be effectively separated from the related substances.
Owner:ANHUI YELLEN PHARMA
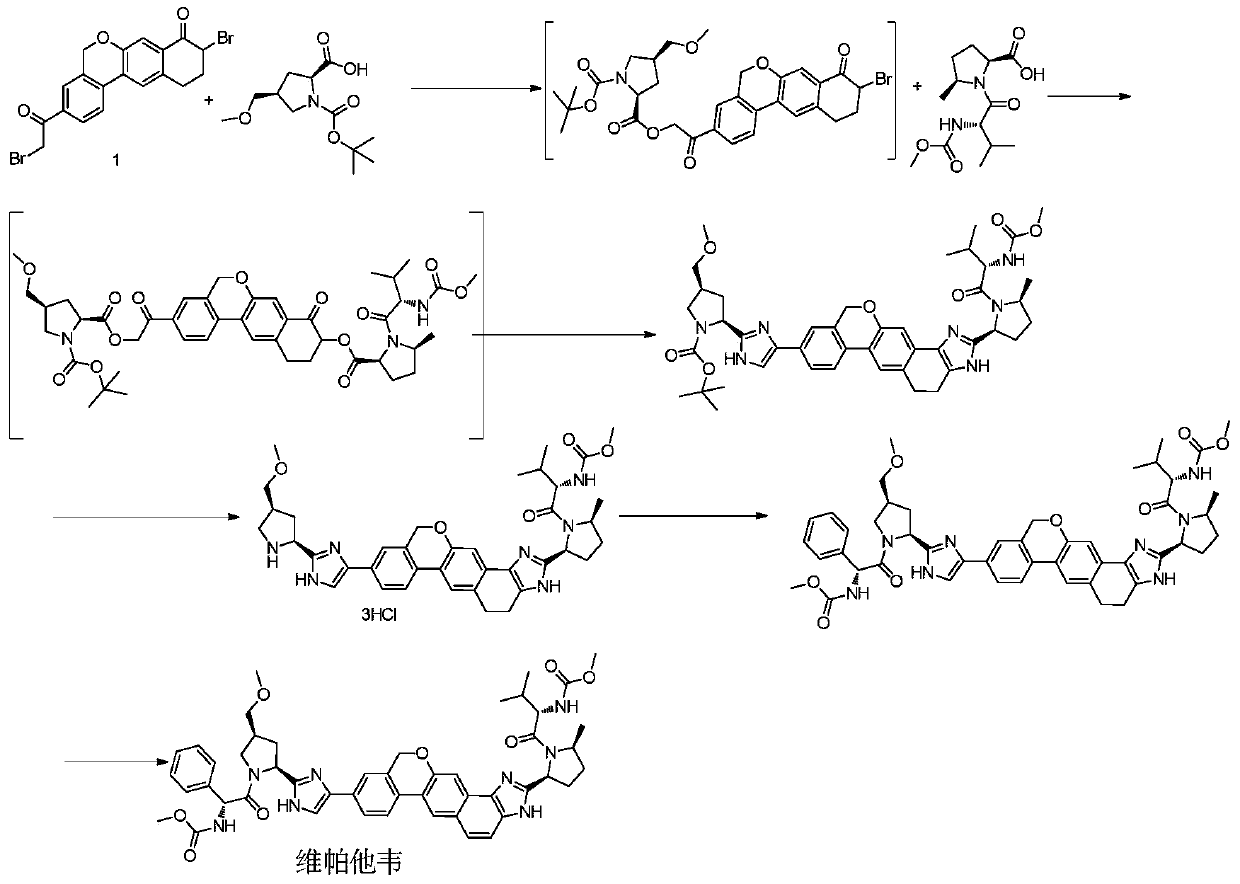
A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative
ActiveCN106916134BThe reaction steps are easy to controlStable industrial production preparationOrganic chemistryChlorobenzeneSodium cyanide
The invention discloses a preparation method of an anti-hepatitis C medicine Velpatasvir intermediate 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative which is 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one (V). The preparation method concretely comprises the following steps: carrying out coupling cyclization on a raw material 7-((2-bromo-5-chlorophenyl)oxo)-3,4-dihydronaphthalen-1(2H)-one under the action of a ligand and an alkali to synthesize 3-chloro-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one, substituting chlorine by using sodium cyanide to form cyanide, prearing an organic acid from the cyanide under the action of an alkali, processing the organic acid by a lithium methide reagent to obtain 3-acetyl-10,11-dihydro-5H-benzo[d]naphtho[2,3-b] ]pyran-8(9H)-one, and carrying out a two-step bromination reaction to obtain the anti-hepatitis C medicine Velpatasvir intermediate. The method provides a brand new synthesis route of the anti-hepatitis C medicine Velpatasvir intermediate, and has the advantages of easily controlled reaction steps, and realization of stable industrial production preparation.
Owner:苏州楚凯药业有限公司
A kind of preparation method of velpatasvir intermediate and its analog
ActiveCN108147972BAvoid PurificationEasy to operateOrganic compound preparationCarboxylic acid amides preparationBiochemical engineeringCombinatorial chemistry
Disclosed is a preparation method for a compound shown by formula (E), a Velpatasvir intermediate shown by formula (K) and an analogue thereof. The method of the present invention uses inexpensive and easily available materials, and a simple process operation, without the need for separating the intermediates, the products are by column chromatography, and it is suitable for industrialized large-scale production..
Owner:XILING LAB CO LTD
Preparation of velpatasvir and its derivatives
The present invention relates to a preparation method of velpatasvir and its derivatives, specifically, the present invention prepares velpatasvir and its derivatives through the intermediate compound shown in the following formula (the definition of each group is as in the specification mentioned). The method has few by-products and low cost, and is suitable for industrial production of velpatasvir.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
New synthesis method of hepatitis C drug velpatasvir
ActiveCN105732765BIncrease profitEfficient synthesisPeptidesTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention provides a novel synthesis method of hepatitis drug velpatasvir.Two intermediate compounds including a compound 4 and a compound III are utilized to synthesize the velpatasvir, the structures of the two compounds are as shown in the following formulas, wherein PG radical is t-butyloxycarboryl (Boc), carboxybenzyl (Cbz), acetyl, benzoyl or (S)-2-methoxyl acyl carbonyl amino-3-methyl-butyryl group (Moc-L-Valyl).
Owner:山东科巢生物制药有限公司
A kind of recrystallization method of the key intermediate of hepatitis C virus drug velpatasvir
The invention provides a method for re-crystallizing a key intermediate of a hepatitis C virus drug velpatasvir. Highly pure 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8(9H)-one is obtained, and parts of organic solvents can be recovered, so the method is economical and environmentally friendly. The method concretely includes the following steps: dissolving crude 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8(9H)-one in a mixed solvent containing a high-solubility organic solvent A and a low-solubility organic solvent B under a 35-70 DEG Cheating condition, precipitating crystals by a gradient cooling technology, centrifuging the obtained system, carrying out vacuum drying on the obtained wet solid at 30-70 DEG C, and performing normalpressure distillation on the obtained centrifugal mother liquor to recover the solvent B.
Owner:SULI PHARMA TECH JIANGYIN
A kind of preparation method of benzochromene derivative
ActiveCN105801553BHigh yieldSuitable for large-scale industrial productionOrganic compound preparationHydroxy compound preparationBenzeneMedicinal chemistry
The invention discloses a preparation method of a benzochromene derivative shown as a formula (I). The benzochromene derivative can be taken as a synthesis intermediate of a drug such as a synthesis intermediate of Velpatasvir. Cheap and available 2-fluoro halogenated benzene is taken as a starting material, a brand-new synthetic route for preparing the benzochromene derivative is provided, the total yield of the whole reaction route is high, and the method is suitable for large-scale industrial production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone](https://images-eureka.patsnap.com/patent_img/fcd42d00-7772-4241-80a5-001fe742800b/HDA0000947621610000011.PNG)
![Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone](https://images-eureka.patsnap.com/patent_img/fcd42d00-7772-4241-80a5-001fe742800b/BDA0000947621600000021.PNG)
![Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone Novel method for synthesizing 9- bromine-3-(2-bromoacetyl)-10,11-dihydro-5H-dibenzo [c, g] chromene-8(9h)-ketone](https://images-eureka.patsnap.com/patent_img/fcd42d00-7772-4241-80a5-001fe742800b/BDA0000947621600000031.PNG)
![Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative](https://images-eureka.patsnap.com/patent_img/fb8df572-bad4-4031-b06d-1aa5254f07af/DEST_PATH_FDA0000999948060000011.png)
![Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative](https://images-eureka.patsnap.com/patent_img/fb8df572-bad4-4031-b06d-1aa5254f07af/DEST_PATH_FDA0000999948060000012.png)
![Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative Preparation method of 10,11-dihydro-5H-benzo[d]naphtha[2,3-b]pyranone derivative](https://images-eureka.patsnap.com/patent_img/fb8df572-bad4-4031-b06d-1aa5254f07af/DEST_PATH_GDA0000999948070000011.png)
![A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative](https://images-eureka.patsnap.com/patent_img/5dd195e8-e8c0-4bf7-9e30-4d48b683a3f2/DEST_PATH_GDA0000999948070000011.png)
![A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative](https://images-eureka.patsnap.com/patent_img/5dd195e8-e8c0-4bf7-9e30-4d48b683a3f2/DEST_PATH_GDA0000999948070000021.png)
![A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative A kind of preparation method of 10,11-dihydro-5h-benzo[d]naphtho[2,3-b]pyrone derivative](https://images-eureka.patsnap.com/patent_img/5dd195e8-e8c0-4bf7-9e30-4d48b683a3f2/DEST_PATH_GDA0000999948070000022.png)