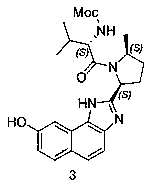
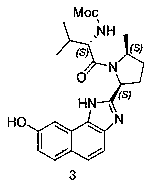
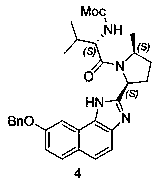
Velpatasvir intermediate and preparation method thereof
A technology for intermediates and compounds, applied in the field of Velpatasvir (GS-5816) intermediates, can solve the problems of inability to process preparation, long reaction routes, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012]
[0013] Add 402g of 2-bromobenzoic acid, 500g of nidosuccinimide, 1L of tetrahydrofuran, and 450g of concentrated sulfuric acid into a 3L reaction flask. Control, iodine color development, raw material points disappear, after concentrating tetrahydrofuran, add methanol 1L, sulfuric acid 250g reflux reaction, TLC (hexane: ethyl acetate 1:2) control, after the intermediate reaction is complete, cool down to 0-5 °C , dropwise added 40% sodium hydroxide to neutralize to neutrality, concentrated the solvent methanol, added ethyl acetate to extract the product, dried over anhydrous magnesium sulfate, concentrated to obtain 610 g of 12a product, 89.5%.
example 2
[0015]
[0016] In a 5L reaction flask, under argon protection, add 610g of methyl 2-bromo-5-iodobenzoate (12a), add 3L of toluene, 380g of potassium acetate, 490g of pinacol borate, 2-dicyclohexylphosphorus-2 35g of '-methylbiphenyl, 20g of palladium acetate, heat up and reflux for 1 hour, TLC (hexane: ethyl acetate 1:2) spot on the central control plate, iodine color develops, the raw material point disappears, cool to room temperature, add potassium phosphate 1200g and 715g iodoimidazole 14a, under the protection of argon, heat up to reflux reaction for 2 hours in the control, TLC (hexane: ethyl acetate 1:2) spot plate in the control, iodine color, the raw material point disappears, cool to At room temperature, separate liquids, add 1L of ethyl acetate to the organic phase to dilute, wash twice with 2L of saturated sodium chloride solution, filter the organic phase with diatomaceous earth, add 100g of N-acetyl-L-cysteine, and stir at room temperature for 12 After 2 hours...
example 3
[0018]
[0019] In a 5L reaction flask, under the protection of nitrogen, add 700g of 11a and 3.5L of anhydrous tetrahydrofuran, cool down to 0 to 5°C, slowly add 70g of lithium aluminum hydride in batches, after the addition is completed, naturally warm up to room temperature and react for 6 hours, add decahydrate sulfuric acid for sampling Sodium quenching, TLC (hexane: ethyl acetate 1:4) spot plate central control, iodine color development, raw material point disappears, add 1200g sodium sulfate decahydrate to quench the reaction, pay attention to temperature control, filter, filter cake with 1L tetrahydrofuran After washing, the filtrates were combined and concentrated to obtain 600 g of product 10a with a yield of 93%, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com