New crystal form of Velpatasvir and preparation method of new crystal form
A technology of crystal form and crystallization treatment, which is applied in the field of co-crystal compound of velpatasvir and saccharin, new crystal form of velpatasvir, which can solve the problems of crystal form stability, purification effect, hygroscopicity and process stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 Preparation of the amorphous substance of the compound of formula I
[0065] Weigh 84 mg of the crude compound of formula II (99% purity) into a 1.5 ml centrifuge tube, add 1 mL of ethyl acetate, sonicate until dissolved, add 40 mg of saccharin and sonicate for 5 minutes, a light yellow oily precipitate forms.
[0066] Centrifuge and remove the supernatant, and dry in an oven at 60° C. for 12 hours to obtain a yellow solid, which is determined to be an amorphous compound of formula I by a polarizing microscope.
Embodiment 2
[0067] Embodiment 2 Preparation of the crystal form A of the compound of formula I
[0068] Weigh 84mg of crude compound of formula II (99% purity) into a 1.5ml centrifuge tube, add 0.4ml of acetone, sonicate until dissolved, then add 38mg of saccharin, sonicate until dissolved. The solution was left at room temperature. After 7 days, a solid precipitate was observed, which was confirmed to be crystal by polarizing microscope.
[0069] Place the centrifuge tube in a centrifuge (Eppendorf minispin) at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour to obtain the co-crystal form A of velpatasvir and saccharin .
[0070] result
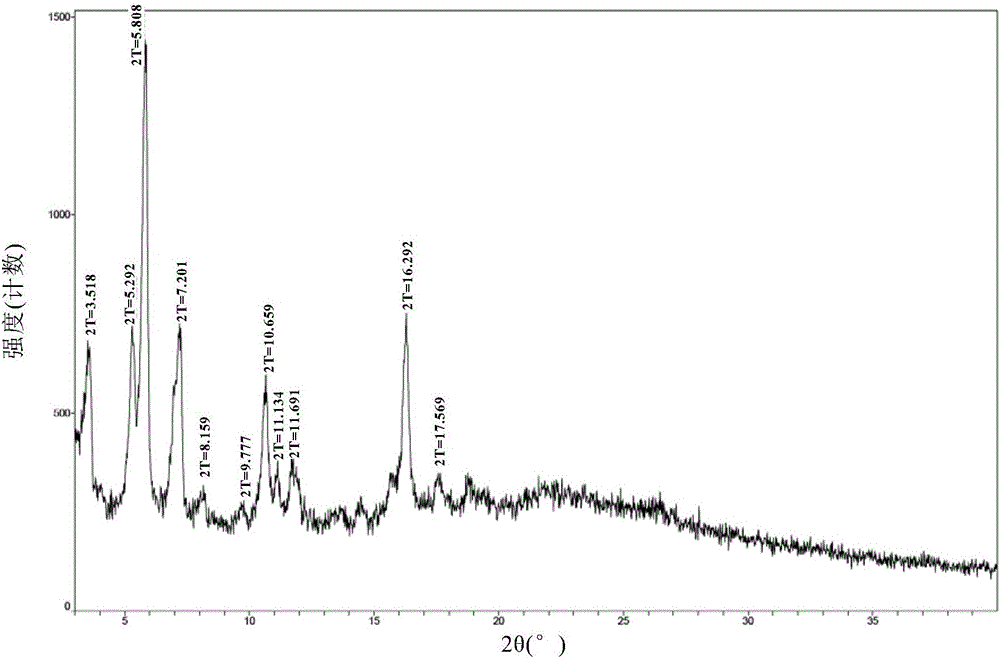
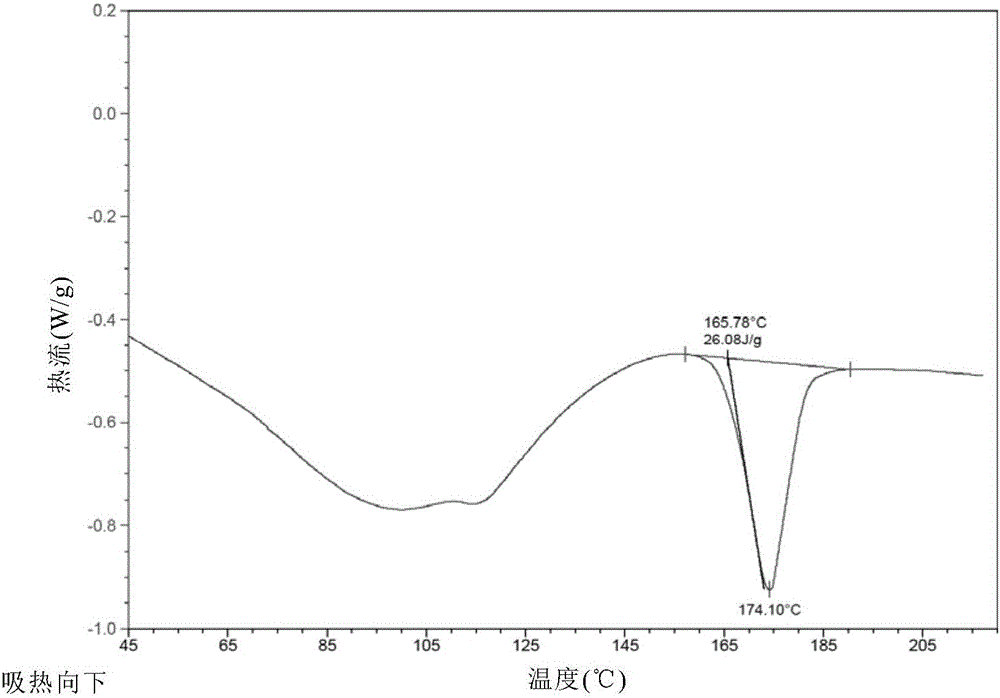
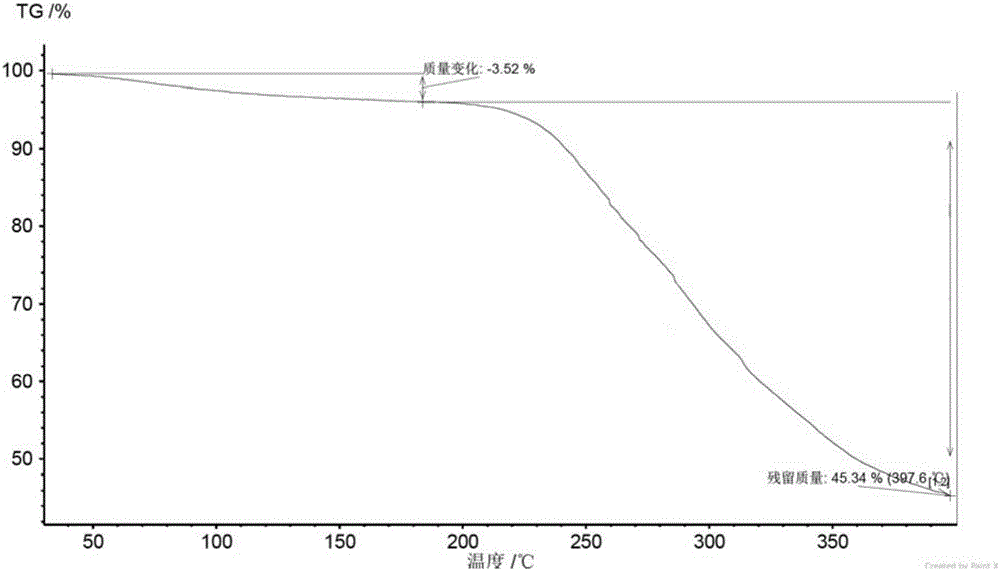
[0071] The crystal form A obtained in Example 2 was detected by XRD, DSC, TGA and HPLC.
[0072] The XRD figure of the formula I compound crystal form A of the embodiment of the present invention 2 is as follows figure 1 , wherein the main diffraction peaks and relative intensities of Form...
Embodiment 3
[0081] Example 3 Preparation of Form A of Formula I Compound
[0082] Weigh 100 mg of the amorphous compound of Formula I (purity 99%) into a 1.5 ml centrifuge tube, add 1 ml of acetone, sonicate until dissolved, then add the seed crystal of Form A, and place the solution at room temperature. Precipitation of a solid was observed after 4 hours.
[0083] Place the centrifuge tube in a centrifuge (Eppendorf minispin) at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour, which is confirmed by XRPD to be a co-crystal of velpatasvir and saccharin Form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com