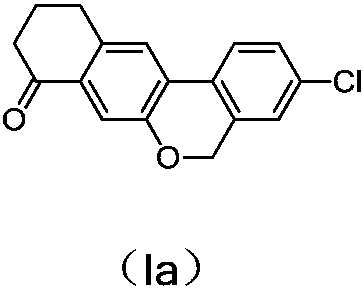
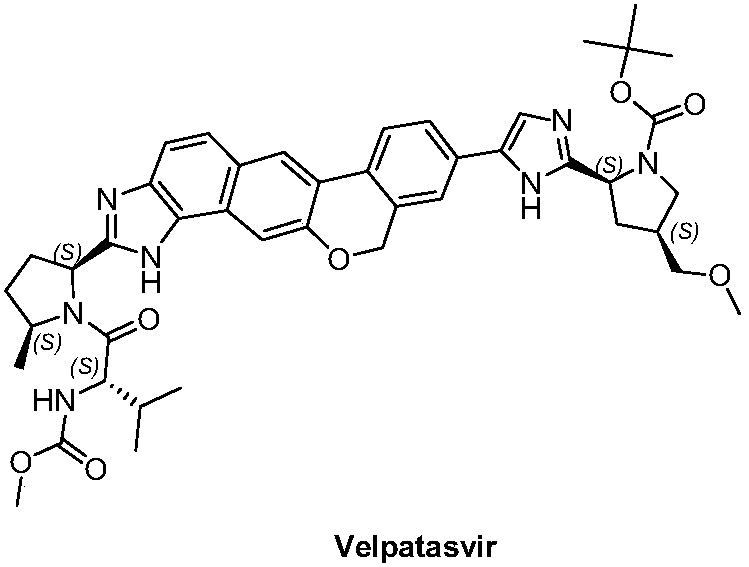
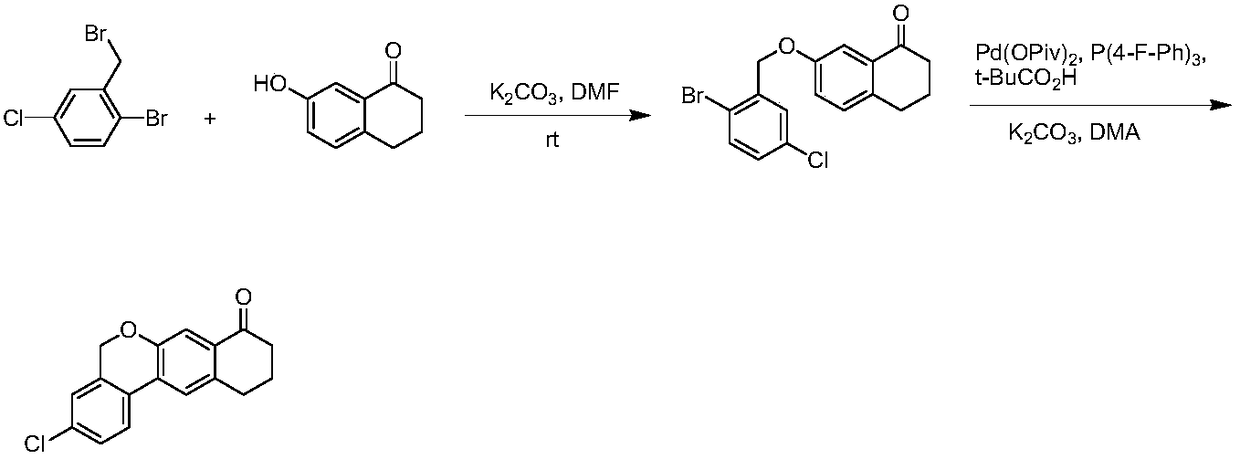
A kind of preparation method of benzochromene derivative
A technology for compounds and mixtures, applied in the field of preparation of benzochromene derivatives, can solve the problems of difficulty in adapting to large-scale industrial production, high price, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of embodiment 1 compound 2
[0094]
[0095] Under the protection of nitrogen, 4 kg (22.9 mol) of compound 1 and 20 L of THF were successively added into a 50 L reaction kettle, and the temperature was lowered to -70° C. Control the temperature below -70°C and add 6.8kg of n-BuLi dropwise, and then stir for 0.5 hours below -70°C after the drop. Control the temperature below -70°C and add 4.75kg of triisopropyl borate dropwise. Pour it into 20L (1N) dilute hydrochloric acid, separate the layers, and extract the aqueous phase with EA10L once more. The organic phases were combined, washed once with 10 L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 2.4 kg of compound 2 with a yield of 85%.
Embodiment 2
[0096] The preparation of embodiment 2 compound 4
[0097] method 1:
[0098]
[0099] Under nitrogen protection, 500 g of compound 3, 3 L of THF, 382 g of compound 2, 626 g of potassium carbonate, 3 L of deionized water, and 6 g of tetrakis(triphenylphosphopalladium) were successively added into a 10 L four-neck flask, and the temperature was raised to reflux for overnight reaction. TLC monitors that the reaction is complete (about 16 hours), and then pours into 3L of ice water, separates the liquid, and extracts the aqueous phase once with EA1L. The organic phases were combined, washed once with 2 L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 482 g of compound 4 with a yield of 96%.
[0100] HNMR (CDCl 3 ,400MHz):δ7.62(s,1H),7.49-7.11(m,6H),4.69(1H,s),4.50(2H,s).
[0101] Method 2:
[0102]
[0103] Add compound 3 50g, THF 300mL, compound 2 38.2g, potassium carbonate 62.6g, deionized water 300mL, Pd(dppf)Cl 2 0.6g...
Embodiment 3
[0105] The preparation of embodiment 3 compound 5
[0106] method 1:
[0107]
[0108] Under nitrogen protection, 50 g of compound 4, 500 mL of DMSO, and 35.6 g of potassium tert-butoxide were sequentially added into a 1 L four-neck flask, and the temperature was raised to 60° C. for 1 hour. The disappearance of the starting material was monitored by TLC, poured into 1L of ice water, and extracted with MTBE 1L x 3. The organic phases were combined, washed once with 1 L of water and once with 1 L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 37.5 g of compound with a yield of 82%, HPLC=90%.
[0109] Method 2:
[0110]
[0111] Under nitrogen protection, 50 g of compound 4, 500 mL of DMF, K 2 CO 3 43.7g, heated up to 60°C and reacted for 1 hour. The disappearance of the starting material was monitored by TLC, poured into 1L of ice water, and extracted with MTBE 1L x 3. The organic phases were combined, washed once with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com