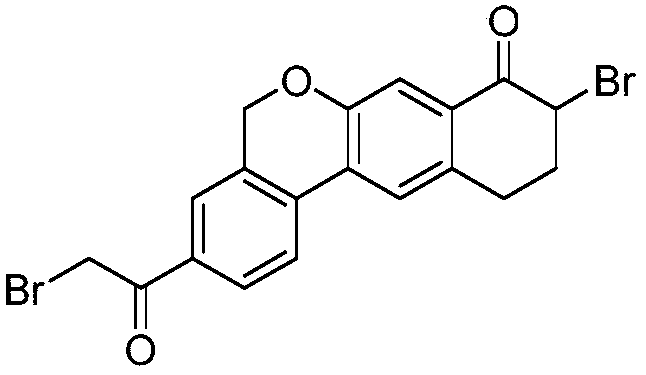
Method for re-crystallizing key intermediate of hepatitis C virus drug velpatasvir
A technology of hepatitis C virus and velpatasvir, applied in the field of medicinal chemistry, can solve the problems of difficult impurity removal, poor solubility of intermediates and by-products, etc., and achieve the effect of high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 5000ml dry reaction flask equipped with a reflux condenser and a stirring device, put in 600ml of N-methylpyrrolidone and 1400ml of acetone, and put in 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H -Benzo[D]naphtho[2,3-B]pyran-8(9H)-ketone crude product 200g, evacuated and replaced with nitrogen, heated to 55-60℃, kept at the temperature and stirred for 2 hours until completely dissolved . Cooling and stirring for crystallization: rotate speed 75-80rpm, about 40-50 minutes, reduce the temperature to 25-30℃, keep stirring for 1 hour, continue for about 40-50 minutes, cool to 5-10℃, keep stirring for 2-3 hours , The filtered wet solid product was vacuum dried at 40-50°C to obtain 182.3 g of light yellow solid with a purity of 99.4% and a yield of 91.2%. The mother liquor was sorted under normal pressure at 75°C, and 1246ml of acetone was recovered. The recovery rate of acetone was 89%.
Embodiment 2
[0027] In a 5000ml dry reaction flask equipped with a reflux condenser and a stirring device, add 500ml of N-methylpyrrolidone and 1500ml of tetrahydrofuran, and add 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H -Benzo[D]naphtho[2,3-B]pyran-8(9H)-ketone crude product 200g, evacuated and replaced with nitrogen, heated to 60-65°C, kept warm and stirred for 2 hours, until completely dissolved . Cooling and stirring for crystallization: rotate speed 75-80rpm, about 40-50 minutes, reduce the temperature to 25-30°C, keep stirring for 1 hour, continue for about 40-50 minutes, cool to 0-5°C, keep stirring for 2-3 hours , Filtered and dried under vacuum at 40-50°C to obtain 178.9 g of light yellow solid with a purity of 99.6% and a yield of 89.5%. The mother liquor was sorted under normal pressure at 75°C, and 1385ml of tetrahydrofuran was recovered. The recovery rate of tetrahydrofuran was 92.3%.
Embodiment 3
[0029] In a 5000ml dry reaction flask equipped with a reflux condenser and a stirring device, add 500ml of N,N-dimethylacetamide and 1500ml of dichloromethane, and add 9-bromo-3-(2-bromoacetyl)-10, 11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8(9H)-one crude product 200 grams, vacuumed and replaced with nitrogen, heated to 35-40°C, kept stirring for 2 Hours, to completely dissolve. Cooling and stirring for crystallization: Rotate speed 60-65rpm, about 40-50 minutes, reduce the temperature to 20-25°C, keep stirring for 1 hour, continue for about 40-50 minutes, cool to 0-5°C, keep stirring for 2-3 hours , Filtered and dried under vacuum at 30-40°C to obtain 183.3 g of pale yellow solid with a purity of 99.6% and a yield of 91.7%. The mother liquor was sorted under normal pressure at 45°C, and 1,215ml of methylene chloride was recovered, and the methylene chloride recovery rate was 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com