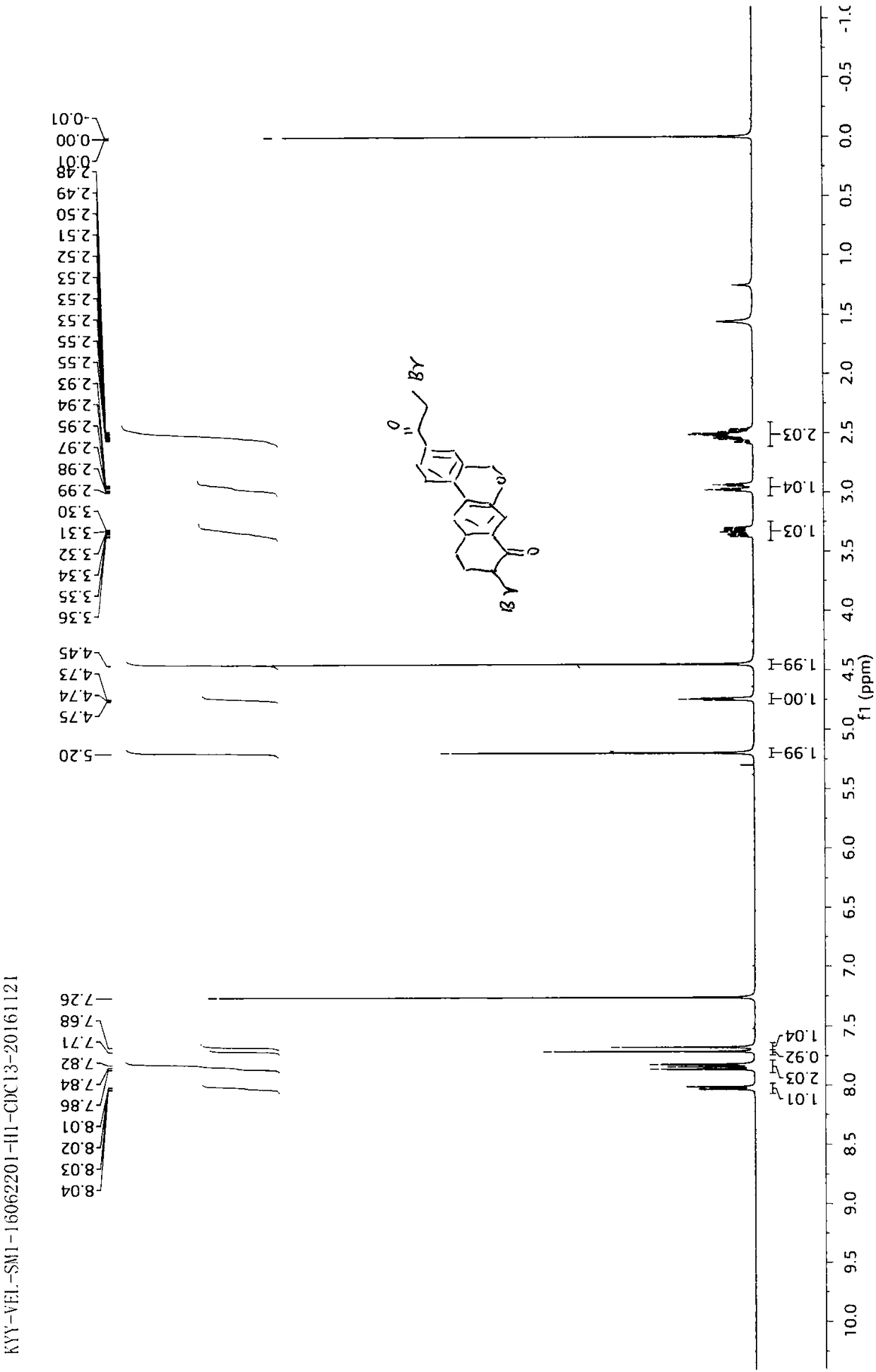
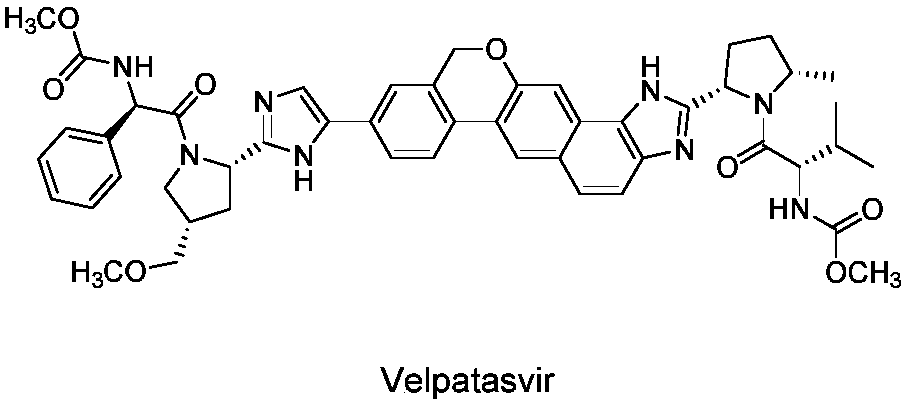
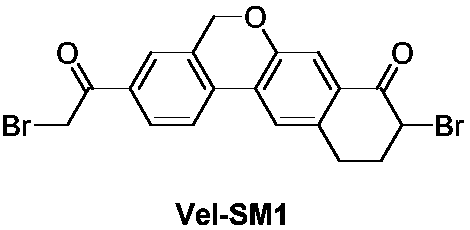
Preparation method of velpatasvir intermediate and analogue thereof
A technology for compounds and mixtures, applied in the field of chemical synthesis, can solve problems such as uneven heating and restricting industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Embodiment 1 prepares o-methylacetanilide
[0125] method 1:
[0126]
[0127] Take 5.0 kg of o-methylaniline, put 30 L of dichloromethane in a 50 L reactor, stir and dissolve, then add 5.2 kg of triethylamine, and slowly add 4.8 kg of acetic anhydride thereinto under ice bath. After addition, slowly rise to room temperature and stir for 2h. 10 L of water was added to the reaction solution and stirred for 10 min, and the organic layer was washed with 2 L of 5% hydrochloric acid solution. Sample HPLC quantification, yield 93.0%, HPLC purity 97.1%. The resulting product was directly subjected to the next reaction without isolation.
[0128] Method 2:
[0129]
[0130] Take 5.0 kg of o-methylaniline and 30 L of dichloromethane in a 50 L reactor, stir and dissolve, then add 5.2 kg of triethylamine, and slowly add 3.7 kg of acetyl chloride dropwise therein under an ice bath. After addition, slowly rise to room temperature and stir for 2h. 10 L of water was added ...
Embodiment 2
[0131] Embodiment 2 prepares 4-bromo-2-methylacetanilide
[0132] method 1:
[0133]
[0134] Take 5.0 kg of o-methylacetanilide obtained in Method 1 of Example 1, put 25 L of acetic acid in a 50 L reactor, add 5.4 kg of elemental bromine dropwise thereto at room temperature, and react at 50°C for 1.5 h after the dropwise addition is completed. After the reaction was completed, 15L of ethyl acetate was added to the reaction solution to dissolve, and then ice-water was added to separate the layers. The aqueous layer was extracted with 2L of ethyl acetate, and the organic layer was combined. The organic layer was washed successively with 2L of saturated sodium sulfite solution and 2L of water. The organic layer was anhydrous. Drying over sodium sulfate, sample HPLC quantification, yield 96.6%, HPLC purity 95.3%. The resulting product was directly subjected to the next reaction without isolation.
[0135] 1 H NMR (300MHz, CDCl 3 ) δ 7.64 (d, J = 8.0 Hz, 1H), 7.30 (s, 2H), ...
Embodiment 3
[0139] Embodiment 3 prepares 4-iodo-2-methylacetanilide
[0140]
[0141] Take 5.0 kg of o-methylacetanilide obtained in Method 2 of Example 1, put 25 L of dichloromethane in a 50 L reaction kettle, add 9.4 kg of iodine element at room temperature, and react with 8.4 kg of sodium bicarbonate at room temperature for 3 hours. Subsequently, 3L of saturated sodium sulfite solution was added to the reaction liquid to quench the reaction, the organic layer was washed with 10L of water, and the organic layer was sampled for HPLC quantification. The yield was 92.1%, and the HPLC purity was 92.5%. The resulting product was directly subjected to the next reaction without isolation. 1 H NMR (300MHz, CDCl 3 ) δ 7.46 (m, 3H), 6.94 (s, 1H), 2.13 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com