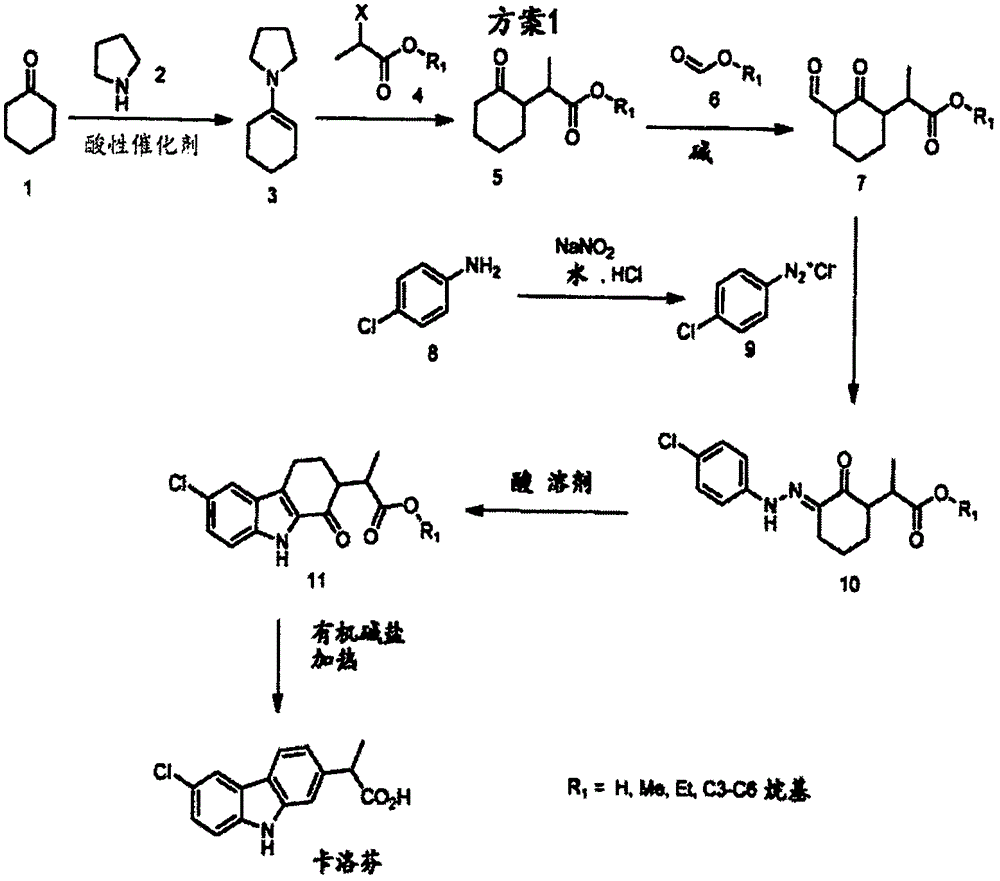
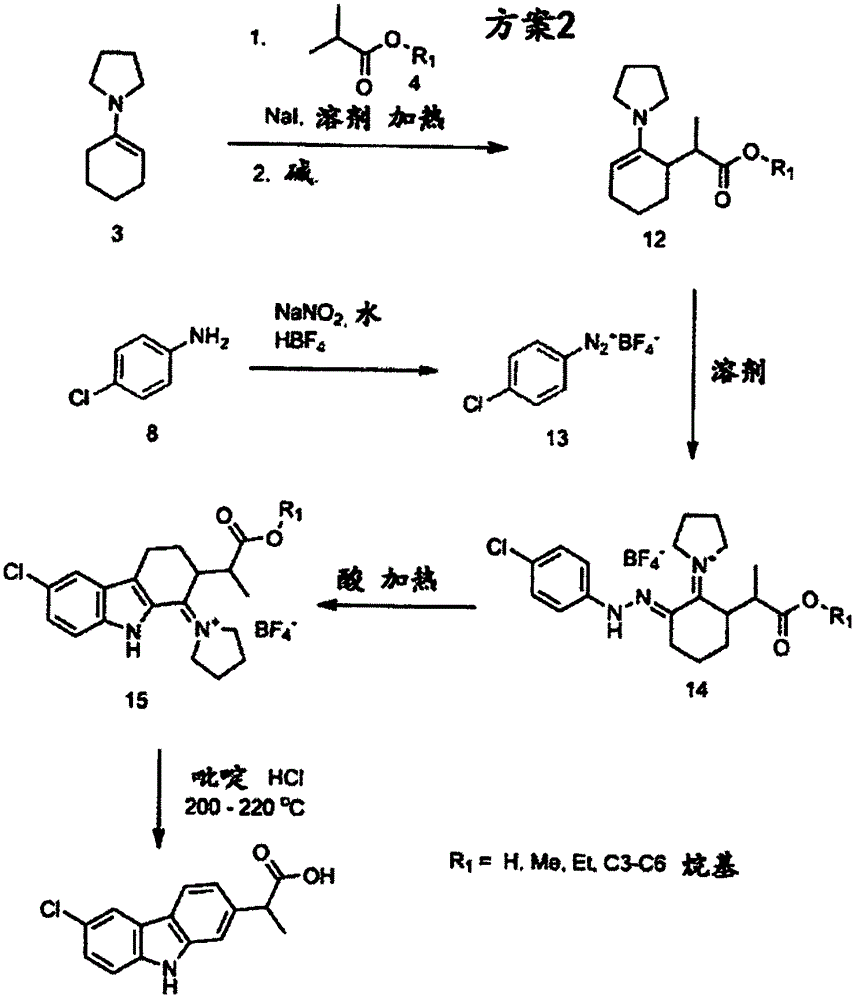
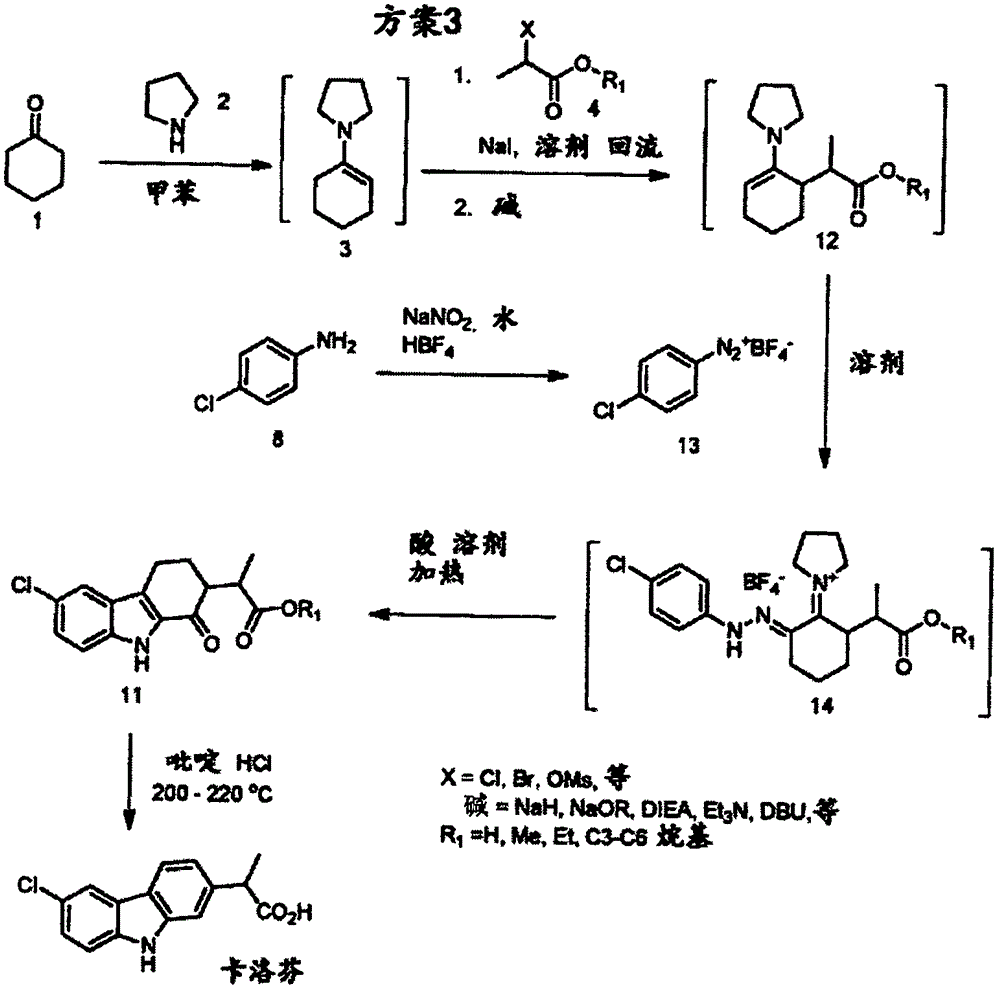
Synthesis method of carprofen
A technology for compounds and mixtures, applied in the field of producing carprofen and its derivatives, can solve problems such as unrealistic industrial production of carprofen, and achieve the effects of easy and cheap acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: 1-(cyclohex-1-en-1-yl)pyrrolidine
[0083] Into a 10 L reactor equipped with a condenser and Dean-stark head was charged cyclohexanone (1449 g), pyrrolidine (1 kg), macroporous sulfonic acid resin (50 g) and toluene (1.5 L). The reaction mixture was heated to reflux and monitored by GC. After 7 h at reflux, GC showed the reaction was complete. The toluene was then removed by evaporation and the residue was vacuum distilled to afford 1-(cyclohex-1-en-1-yl)pyrrolidine, 1673 g (78.6%), 1 HNMR (CDCl 3 ): δ4.26(s, 1H), 2.97(s, 4H), 2.16(s, 2H), 2.07(s, 2H), 1.80(s, 4H), 1.66(m, 2H), 1.53(m, 2H).
Embodiment 2
[0084] Example 2: 2-(2-oxocyclohexyl) methyl propionate
[0085] To a 1 L round bottom flask containing 1-(cyclohex-1-en-1-yl)pyrrolidine (50 g) and methanol (500 mL) was added ethyl 2-bromopropionate (60 g). The reaction mixture was heated to reflux with stirring for 29 hours. Water (60 mL) was then added to the reaction mixture. Reflux was continued for an additional hour. The mixture was then cooled to room temperature before being diluted with water (500 mL). The resulting mixture was extracted with methyl tert-butyl ether (MTBE) (2 x 300 mL). The organic extracts were combined, washed with brine, dried over sodium sulfate and filtered. The filtrate was concentrated. The crude product was vacuum distilled to give the pure product, 7 g (11.5%). 1 HNMR (CDCl 3 ): δ3.66(d, 3H), 2.71-2.59(mm, 2H), 2.32(m, 2H), 2.03(m, 2H), 1.86(m, 1H), 1.62(m, 2H), 1.32( m, 1H), 1.12-1.05 (dd, 3H). MS(ESI)m / z 185.2[M+1] + .
Embodiment 3
[0086] Example 3: Methyl 2(3-formyl-2-oxocyclohexyl)propionate
[0087] A 250 ml round bottom flask was charged with sodium hydride (2.2 g, 60% in mineral oil) under nitrogen, then THF (50 mL) was added dropwise while stirring at room temperature. Then methyl 2-(2-oxocyclohexyl)propionate (5 g) in THF (10 mL), methyl formate (1.6 g), and Anhydrous methanol (0.1 mL) solution. After the addition, the reaction mixture was stirred at room temperature for an additional 5 hours, then treated with water (100 mL). The resulting mixture was extracted twice with methyl tert-butyl ether (MTBE) (2 x 50 mL). The organic extracts were combined, washed with brine, dried over sodium sulfate, filtered and concentrated. The crude product was then purified by column chromatography (petroleum ether: ethyl acetate = 10:1) to give 1 g (17%) of pure product. 1 HNMR (CDCl 3 ): δ8.26(dd, 1H), 3.65(d, 3H), 3.10(m, 1H), 2.90-2.75(mm, 1H), 2.26(m, 2H), 1.86(m, 2H), 1.72( m, 1H), 1.47 (m, 2H), 1.18-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com