Diflufenican synthesis method
A technology of diflufenapyr and a synthesis method, applied in the field of fenflufen synthesis, can solve the problems of unfavorable fenflufen production yield, increased production cost and high production cost of diflufen The effect of purification treatment, increasing reaction rate and ensuring product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
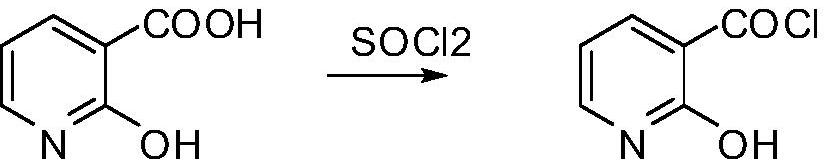
[0044] Put 42g (0.3mol) of 2-hydroxynicotinic acid into a 500mL four-neck flask, add 200mL of toluene, stir and raise the temperature, add 43g (0.36mol) of thionyl chloride dropwise at 80°C, drop it for 1 hour, and react under reflux 1h, distill off 100mL of solvent to obtain 2-hydroxynicotinyl chloride solution for use;
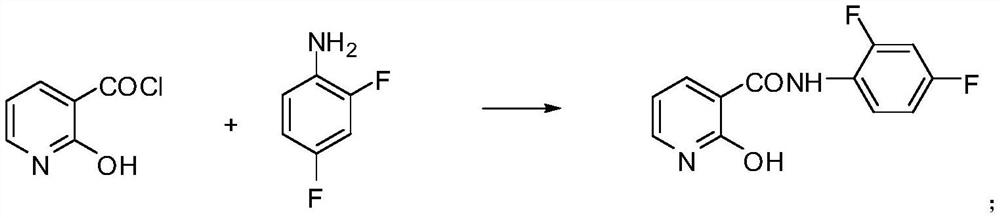
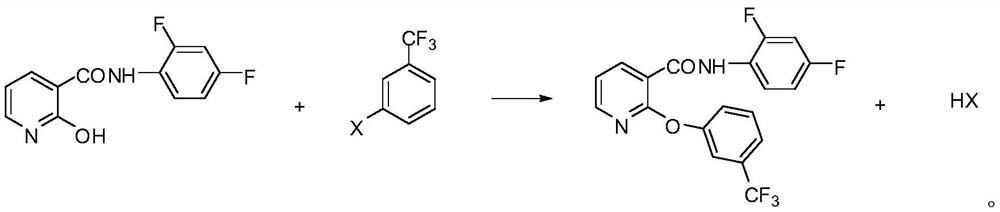
[0045]Put 38.7g (0.297mol) of 2,4-difluoroaniline and 160mL of toluene into a 500mL four-neck flask, stir and heat to 50°C, add the prepared 2-hydroxynicotinoyl chloride solution dropwise, and control the dropping temperature at 50°C. After 30 minutes of dripping, keep the temperature at 60°C for 1 hour, heat up and reflux for 1.5 hours, after the reaction is over, cool down to 100°C, add 42.8g of potassium carbonate and 0.5g of polyethylene glycol, heat up and reflux for dehydration, and add m-trifluoromethyl after the dehydration is completed Chlorobenzene 56.6g (0.31mol), reflux reaction at 110°C for 2.5 hours, after the reaction was completed, heat filte...
Embodiment 2
[0047] Put 42g (0.3mol) of 2-hydroxynicotinic acid into a 500mL four-neck flask, add 200mL of toluene, stir and raise the temperature, add 43g (0.36mol) of thionyl chloride dropwise at 70°C, drop it for 1 hour, and react under reflux 1.5h, sampling analysis, 2-hydroxynicotinic acid 0.4%, acid chloride content 99.2%, distill off 100mL of solvent to obtain 2-hydroxynicotinyl chloride solution for use;
[0048] Put 38.7g (0.297mol) of 2,4-difluoroaniline and 160mL of toluene into a 500mL four-neck flask, stir and heat to 55°C, add the prepared 2-hydroxynicotinoyl chloride solution dropwise, and control the dropping temperature at 55°C. After 30 minutes of dripping, keep warm at 60°C for 1 hour, heat up and reflux for 2 hours, the material is transparent liquid, sample analysis, N-(2,4-difluorophenyl)-2-hydroxy-nicotinamide content is 98.5%, the reaction is over, cool down To 100°C, add 41.5g (0.3mol) of potassium carbonate and 0.5g of polyethylene glycol, heat up and reflux for d...
Embodiment 3
[0050] Put 42g (0.3mol) of 2-hydroxynicotinic acid into a 500mL four-neck flask, add 200mL of toluene, stir and raise the temperature, add 43g (0.36mol) of thionyl chloride dropwise at 85°C, drop it for 1 hour, and react under reflux 2h, distill off 100mL of solvent to obtain 2-hydroxynicotinyl chloride solution for use;
[0051] Put 38.7g (0.297mol) of 2,4-difluoroaniline and 160mL of toluene into a 500mL four-neck flask, stir and heat to 60°C, add the prepared 2-hydroxynicotinoyl chloride solution dropwise, and control the dropping temperature at 60°C. After 30 minutes of dripping, keep warm at 60°C for 1 hour, heat up and reflux for 2.5 hours, the material is transparent liquid, after the reaction is completed, cool down to 100°C, add 46.8g of potassium carbonate and 0.5g of polyethylene glycol, heat up and reflux for dehydration, and add m-trifluoromethylchlorobenzene 56.6g (0.31mol), 110 ℃ reflux reaction for 3.5h, the reaction is completed, hot filtration, the filtrate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com