New velpatasvir intermediate crystal form
A technology of crystal forms and polymorphs, which is applied in the field of new crystal forms of velpatasvir intermediates, can solve the problems of difficult formula I compounds and poor crystallinity of formula I compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The polymorphic form of the present invention can be prepared by the following preparation method, which includes the steps of: mixing the compound of formula I and an inert solvent, and placing it, so as to obtain the polymorphic form of the present invention; wherein, The inert solvent is selected from the following group: toluene, isopropyl ether or a mixed solvent of the two.
[0061] The compound of formula I may be other polymorphs or amorphous form of the compound of formula I.
[0062] In another preferred example, the purity of the compound of formula I is ≤92%.
[0063] In another preferred example, the weight and volume ratio (g / mL) of the compound of formula I to the inert solvent is 1:0.5-1:100; preferably, 1:1-1:20.
[0064] In another preferred embodiment, after the mixing, the resulting mixture is a solution or a suspension.
[0065] In another preferred example, the standing is for 8-72 hours; preferably, for 12-48 hours; more preferably, for 12-24 hour...
Embodiment 1
[0086] The preparation of embodiment 1 crystal form I
[0087] (1) Weigh 74 mg of the compound of formula I (HPLC purity: 91.6%) into a 1.5 ml centrifuge tube, and add 0.1 mL of toluene. Sonicate until dissolved. After standing at room temperature for 16 hours, a solid precipitated out. Part of the solid precipitate was taken and identified as crystal by polarizing microscope.
[0088] (2) Place the centrifuge tube in a centrifuge, centrifuge at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour.
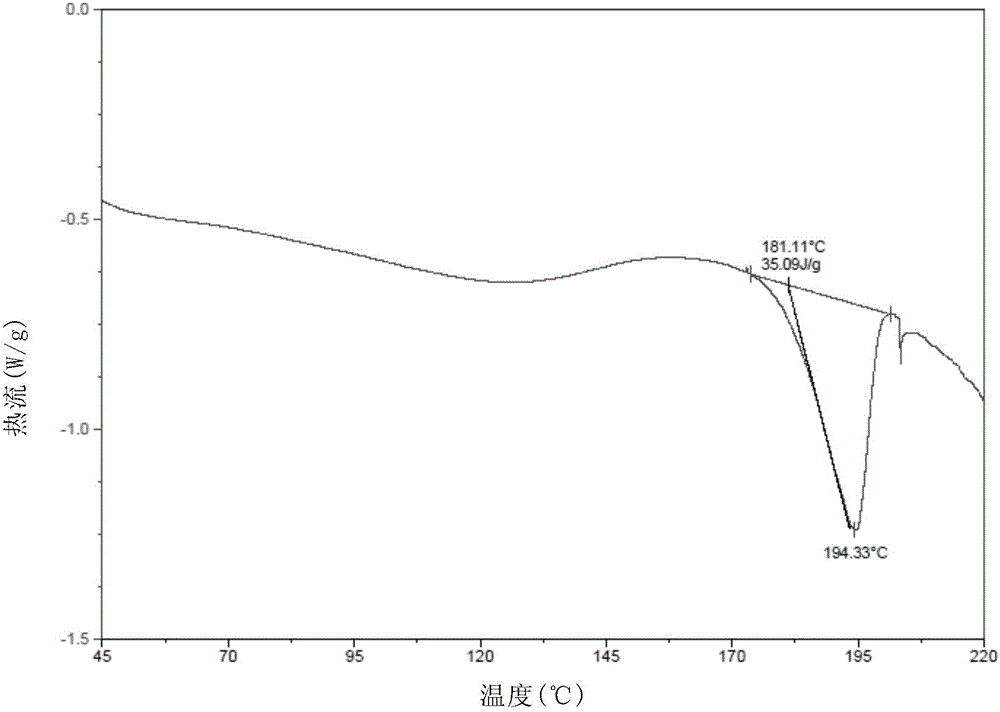
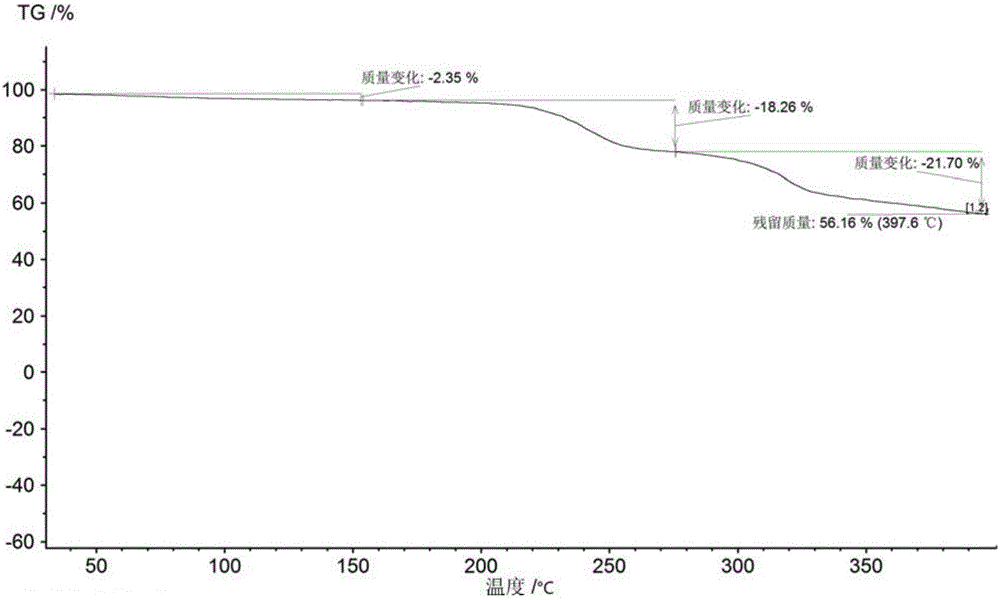
[0089] (3) Characterize the solid form of the dried solid by XRPD, DSC and TGA.
[0090] result
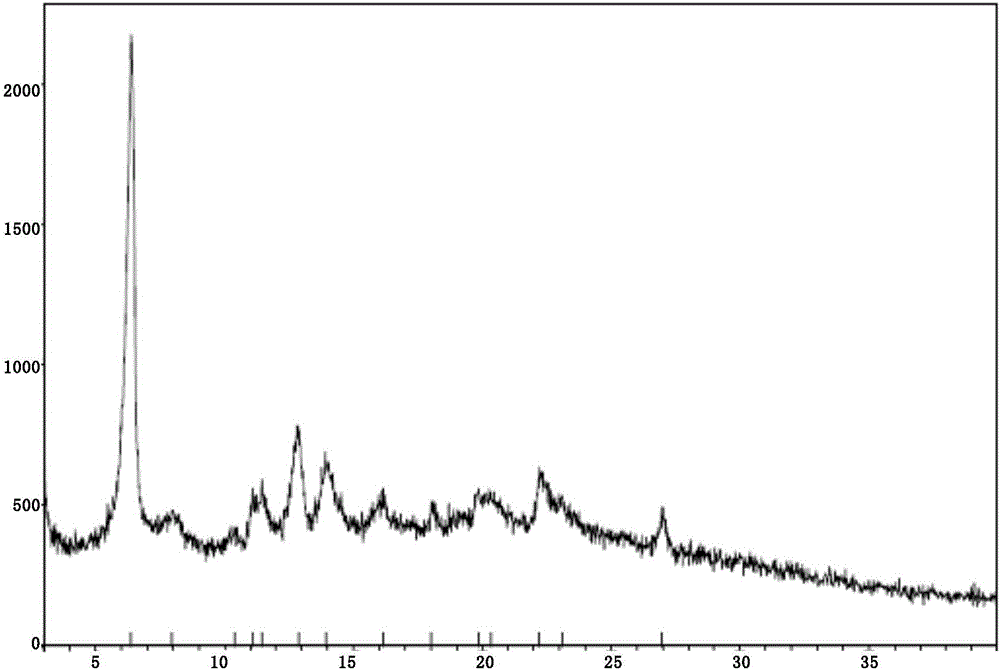
[0091] (1) XRPD pattern
[0092] The crystal of the compound of formula I is named as crystal form I, and its XRPD pattern is as follows: figure 1 shown. The main diffraction peaks and their relative intensities are shown in Table 1.
[0093] Table 1 XRPD data of the crystal
[0094] 2θ(°)
Relative Strength(...
Embodiment 2
[0102] (1) Weigh 74 mg of the compound of formula I (HPLC purity: 91.6%) into a 1.5 ml centrifuge tube, and add 0.1 mL of isopropyl ether. Sonication forms a suspension. After standing at room temperature for 16 hours, a part of the solid precipitate was taken and identified by a polarizing microscope, which was confirmed to be a crystal.
[0103] (2) Place the centrifuge tube in a centrifuge, centrifuge at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour.
[0104] (3) Characterize the solid form of the dried solid by XRPD.
[0105] result
[0106] (1) XRPD pattern
[0107] The XRPD spectrum of the crystal obtained in Comparative Example 2 is consistent with the crystal form I XRPD spectrum, and it is confirmed to be crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com