Combination formulation of three antiviral compounds
A composition and polymer technology, applied in antiviral agents, drug combinations, medical preparations of non-active ingredients, etc., can solve the problem of not giving velpatasvir, sofosbuvir and vociprevir treatment benefits, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0411] Example 1: Tablet Preparation and Formulation
[0412] A. Dose Selection of Tablets
[0413] i. Sofosbuvir
[0414] Sofosbuvir exhibits a variety of forms, including its crystalline forms and solvates (see, eg, US Patent Application Publication Nos: 2010 / 0298257, 2011 / 0251152, and 2015 / 0175646). In some embodiments and the examples provided herein, the tablet formulation includes sofosbuvir in Form 6 and optionally trace amounts of Form 7. Crystalline forms 6 and 7 of sofosbuvir are non-hygroscopic and have similar water solubility of > 2 mg / mL at 37°C. Form 6 of sofosbuvir is the thermodynamically stable form and remains physically stable when suspended in water. Form 7 of sofosbuvir, although physically stable in water for short periods of time, can be converted to Form 6 under prolonged agitation. Additionally, Forms 6 and 7 were not photosensitivity and remained physically and chemically stable when stored in an open container at 40°C / 75%RH for 1 month.
[0415...
Embodiment 2
[0527] Example 2: Chemical and Physical Stability
[0528] The chemical stability, physical stability and dissolution properties of Sofosbuvir / Velpatasvir SSD / Vociprevir SSD Tablets (Formulation G) were evaluated and summarized in Tables 11A, 11B. In some embodiments, film-coated tablets are packaged in 75 mL and 100 mL white HDPE bottles (lots A and B, respectively, as described in Tables 11A, 11B) with 1 g silica gel desiccant canisters and polyester fiber rings. )middle. The 75mL and 100mL HDPE bottles contain 14 and 28 tablets, respectively. Each HDPE bottle is capped with a white continuous thread, child-resistant polypropylene screw cap with an aluminum thread leading into the seal. The packaged tablets (14 and 28 counts) were evaluated for stability at 40°C / 75%RH and 25°C / 60%RH. In addition, the stability of 14-count bottles (without caps) was evaluated under open conditions of 40°C / 75%RH.
[0529] Table 11A
[0530]
[0531]
[0532] a Dissolution condition...
Embodiment 3
[0582] Embodiment 3: physicochemical properties and biological properties
[0583] A. Dissolution as a function of medium pH and composition
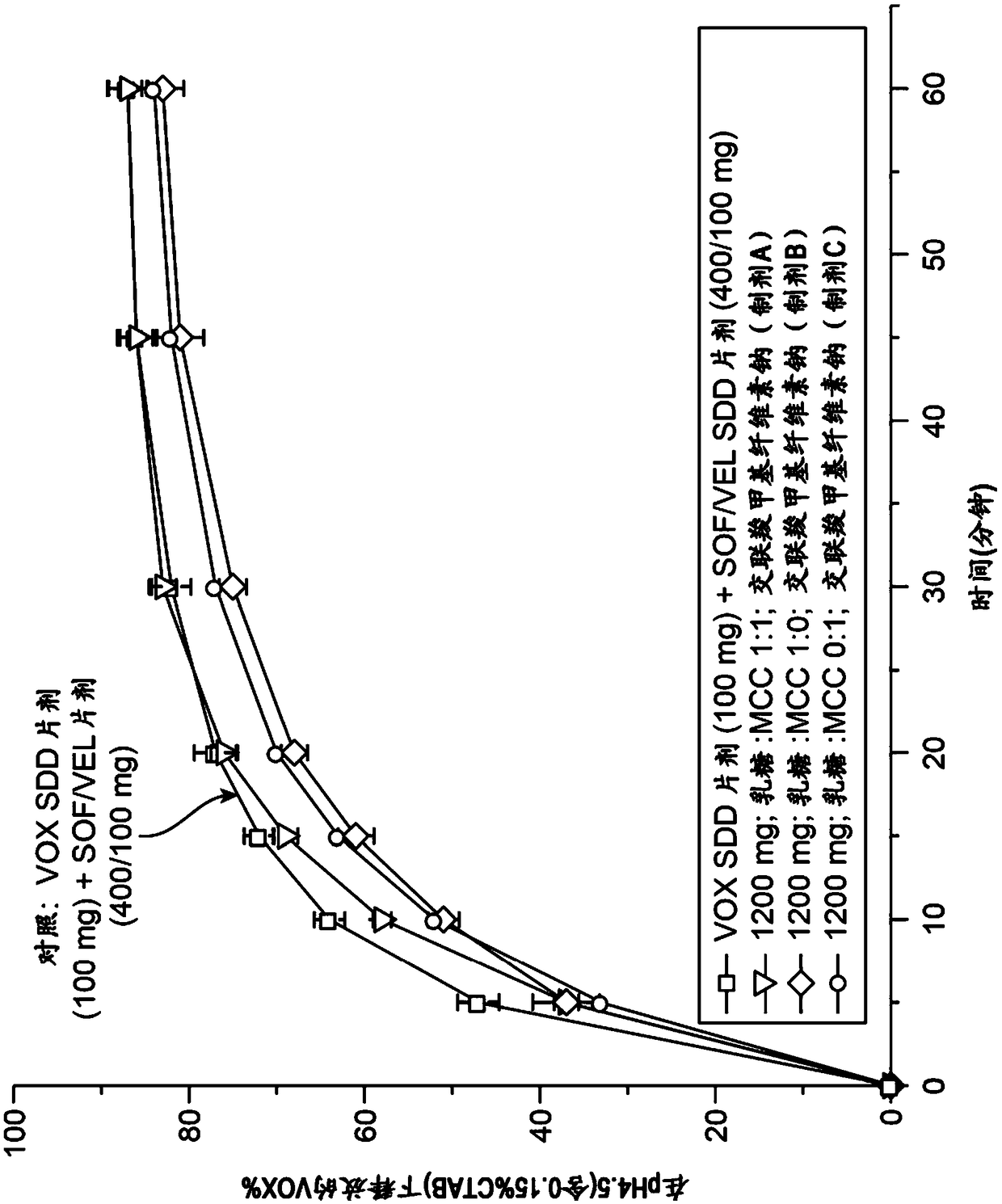
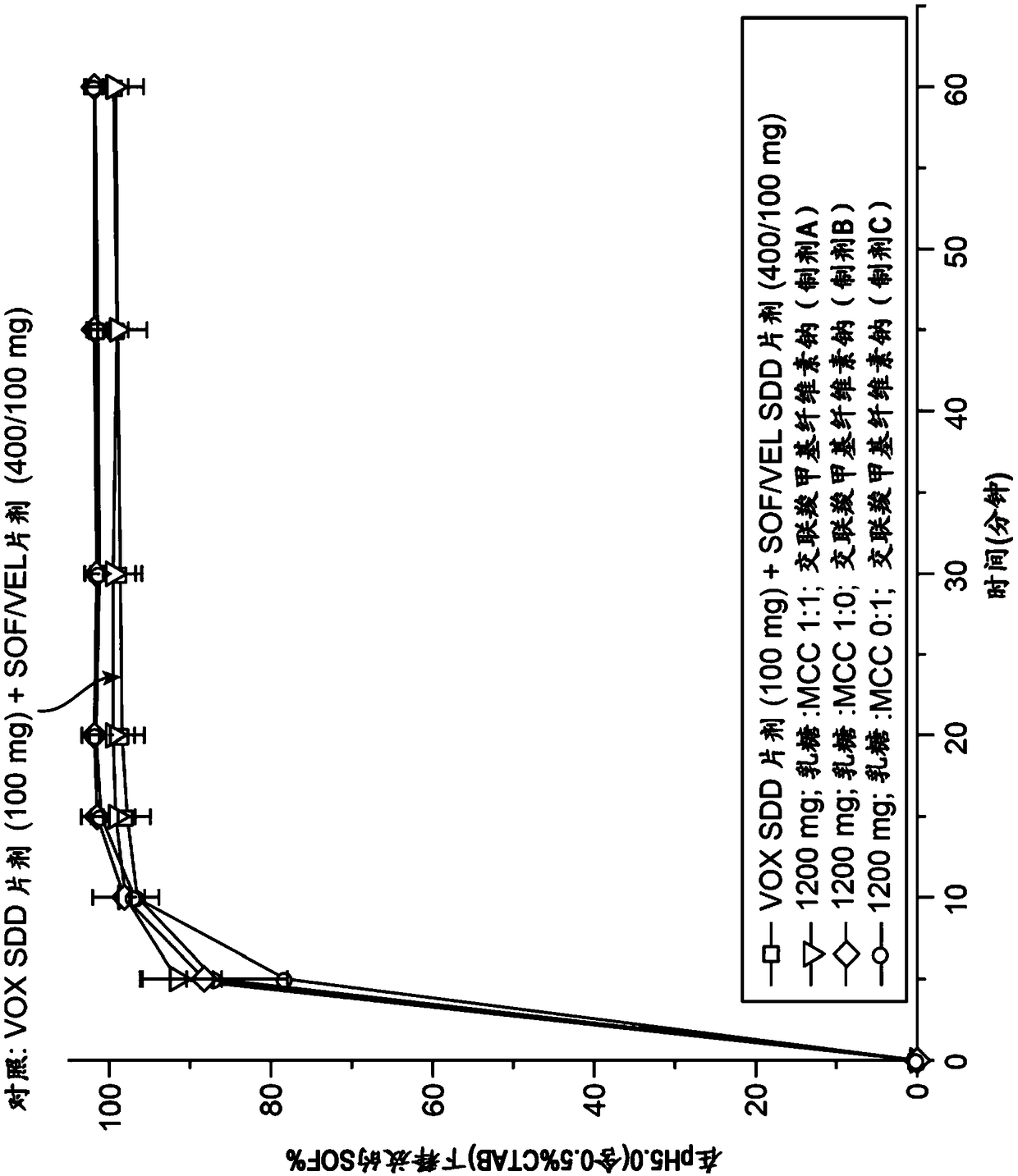
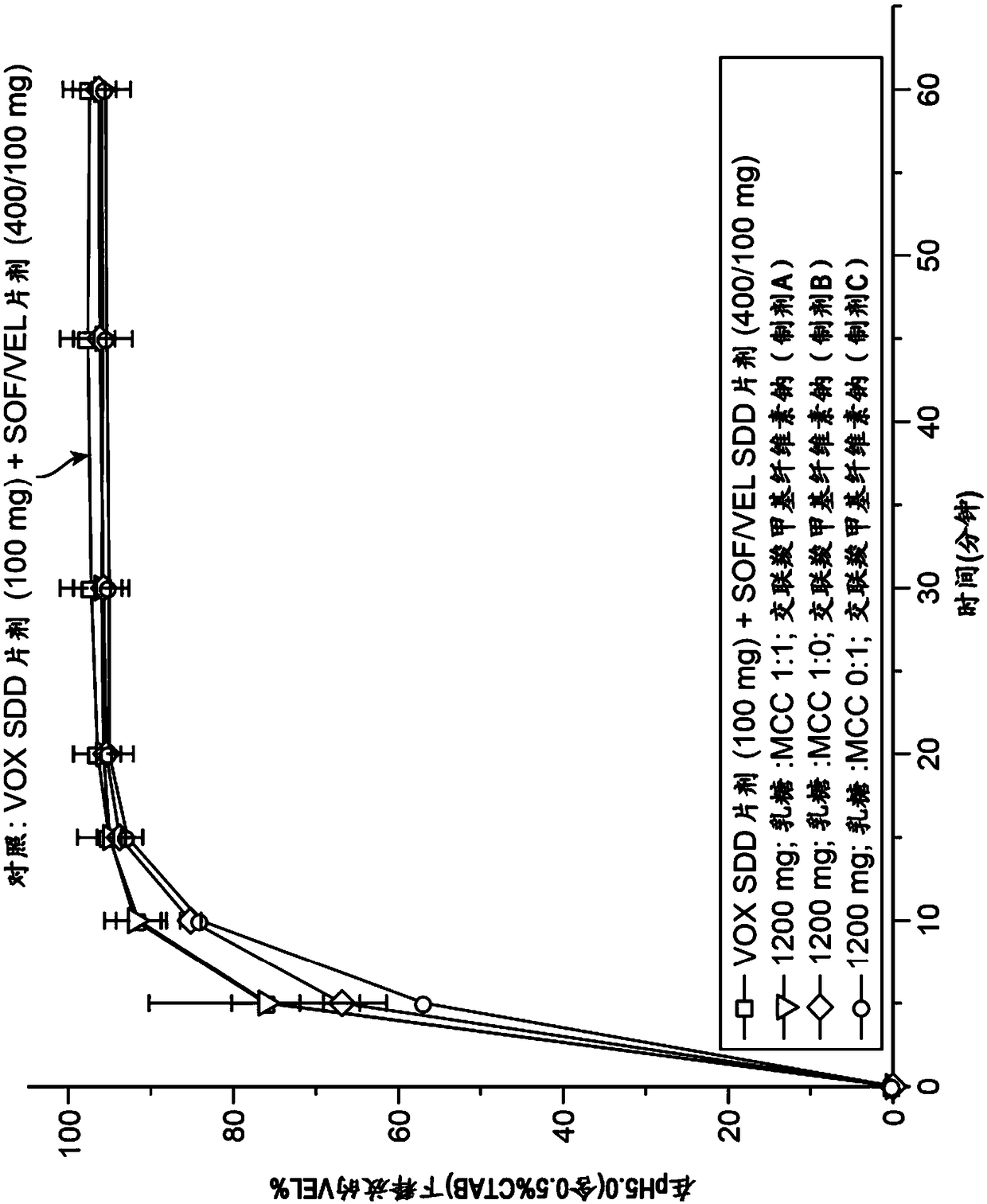
[0584] Obtain Sofosbuvir, Velpatasvir and Vociprevir from Sofosbuvir / Velpatasvir SSD / Vosciprevir SSD using Apparatus II (paddle), 75 rpm, with 900 mL medium at 37°C The dissolution profile of the tablet (formulation G comprising 30.77w / w% sofosbuvir, 15.38w / w% velpatasvir SSD, 15.38w / w% vociprevir SSD) was used as pH in various media The function( Figure 13-15 ). Sofosbuvir is a BCS class 3 compound with high and pH-independent water solubility. Velpatasvir is a BCS class 4 compound with pH-dependent solubility. Vociprevir is a BCS class 2 compound with pH-dependent solubility.
[0585] Such as Figure 13 As shown, the dissolution of sofosbuvir was completed within 20 minutes and was independent of medium pH and composition.
[0586] Such as Figure 14As shown, the dissolution of velpatasvir from sofosbuvir / velpatasvir SSD / voci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com