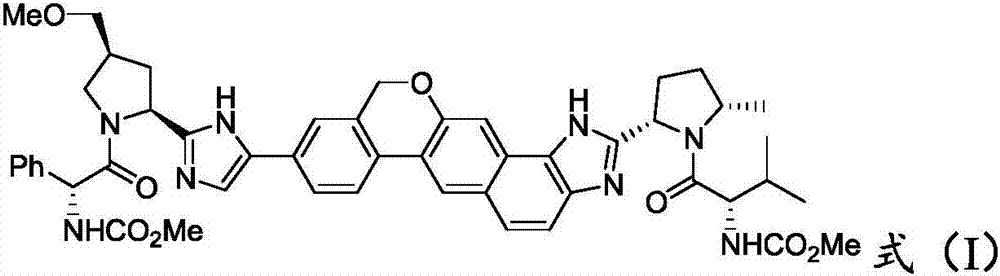
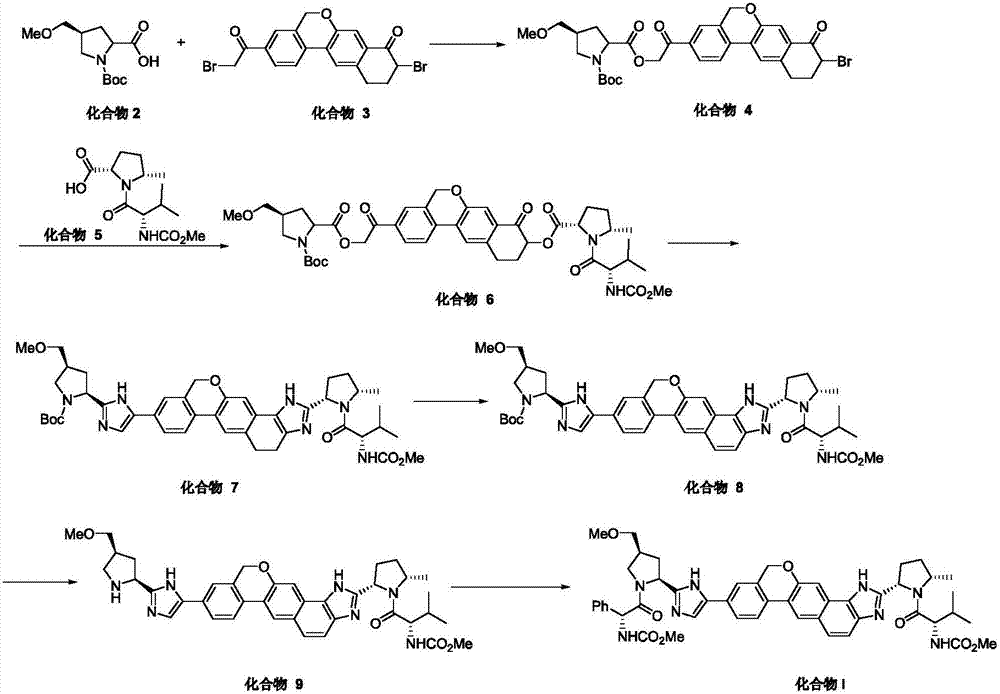
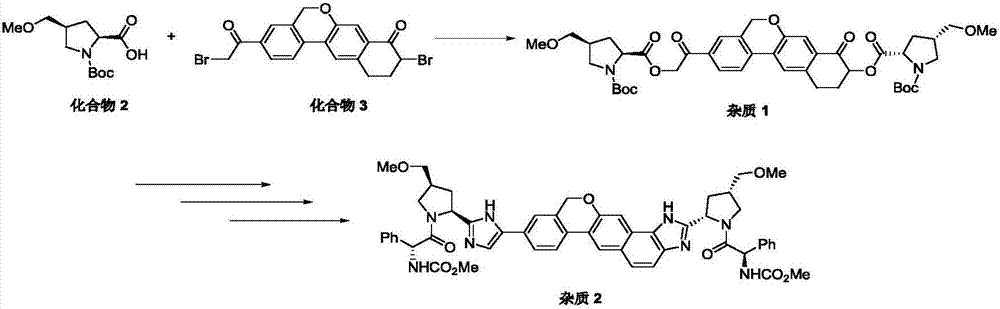
Synthesis method of velpatasvir
A synthesis method and velpatasvir technology, applied in the field of velpatasvir synthesis, can solve problems such as difficult removal of impurities, and achieve the effect of simplifying the post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention provides a synthetic method of velpatasvir, comprising:
[0046] 1) reacting a compound of formula (II) with a compound of formula (III) to obtain a compound of formula (IV);
[0047]
[0048] Wherein, R is tert-butoxycarbonyl or formula (R-a),
[0049]
[0050] 2) converting the compound of formula (IV) into a compound of formula (I);
[0051]
[0052] According to the present invention, the present invention reacts the compound of formula (II) structure with the compound of formula (III) structure, obtains the compound of formula (IV) structure; Wherein, the solvent of described reaction is preferably polar solvent, more preferably di One or more of oxygen hexane, N, N-dimethylacetamide, N-methylpyrrolidone and ethylene glycol dimethyl ether; also add alkaline substance, palladium catalyst and organophosphorus complex in the reaction body; wherein, the alkaline substance is preferably one or more of potassium carbonate, potassium tert-butoxide...
Embodiment 1
[0071] The reaction process is as follows:
[0072]
[0073] Concrete reaction steps are:
[0074] 1.1 Synthesis of Compound 15
[0075] Add 50.0 g of compound 14 and 250 mL of methanol into a 1.0 L reaction flask, and then add 55.0 g of concentrated hydrochloric acid dropwise under stirring. After the dropwise addition, the temperature was raised to 55-60° C., and the reaction was stirred for 12 hours. TLC (PE:EA=1:1, Rf=0.1) showed that the reaction was complete. At 50°C, it was concentrated to dryness under reduced pressure. Add an appropriate amount of methanol and steam twice to obtain 57.0 g of the product. The yield is 100%, and it is directly used in the next step reaction.
[0076] 1.2 Synthesis of Compound 16 (Formula V)
[0077] 61 g of CDMT (2-chloro-4,6-dimethoxy-1,3,5-triazine) and 400 mL of methanol were added to a 1.0 L reaction flask. Under the protection of nitrogen, use an ice bath to cool down to 0-5°C, add 81g of N-methylmorpholine quickly, and s...
Embodiment 2
[0099] The reaction process is:
[0100]
[0101] Specifically synthesized as:
[0102] 2.1 Synthesis of Compound 23 (Formula III-a)
[0103] Add 7.0 g of compound 22 (formula VI-a) and 70 mL of tetrahydrofuran into a 250 mL three-necked flask. Under the protection of nitrogen, cool down to 0-5°C with an ice bath. Then, 1.0 g of sodium hydrogen was added in batches, and the feeding was controlled at a temperature of 0-10°C. After the addition, the reaction was stirred for half an hour at 0-10°C. 2.6 g of 2-(trimethylsilyl)ethoxymethyl chloride was added dropwise, and after the dropwise addition was completed, the reaction was stirred for three hours. Sampling was sent to LC-MS detection to show that the reaction was complete. Add 70 mL of saturated ammonium chloride solution dropwise to quench the reaction (a large number of bubbles are generated), and extract three times with ethyl acetate (150 mL×3). The organic phases were combined, washed twice with 100 mL of satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com