Preparation method of key intermediate of anti-hepatitis C drug ledipasvir
An intermediate and key technology, applied in the field of preparation of 1--2-chloroethyl ketone, can solve the problems of long reaction route, expensive raw materials, low yield, etc., and achieve the effect of mild reaction conditions and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
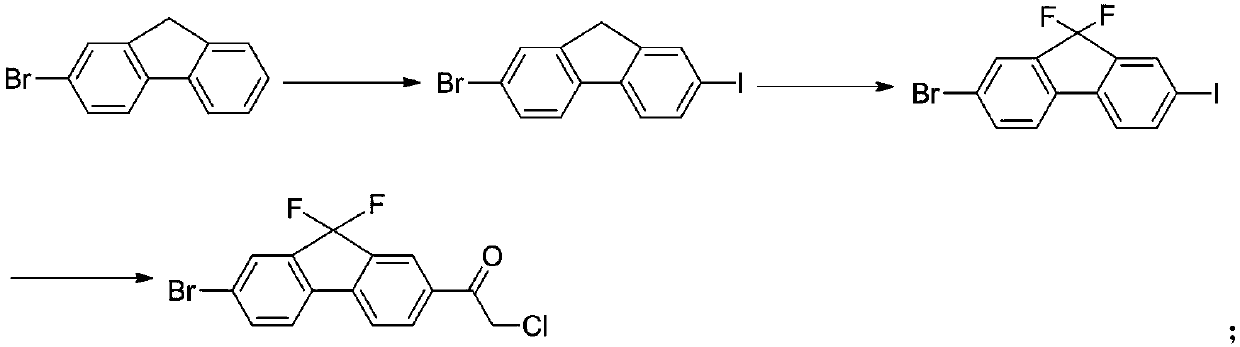
[0053]Synthesis (2) of embodiment 1,2-iodo-5-bromobenzoic acid
[0054] Take 2-amino-5-bromobenzoic acid (2.14g, 10mmol), NaNO 2 (0.828g, 12mmol) and NaOH (0.55g, 11mmol) were dissolved in 40ml of water, under stirring, cooled in an ice bath to 0°C, and 12ml of 6mol / L hydrochloric acid solution was added dropwise, and the drop was completed in 2h. After the hydrochloric acid was added dropwise, the reaction system continued to react at 0°C for 1h, then the reaction system was heated to 35-40°C, and the solution of KI [(KI 2.5g, 15mmol), H2SO4 (0.6ml) and water (5ml) was slowly added ], 20min to finish adding. Then the temperature of the reaction system was raised to 90° C. for 1 h. After the reaction, the reaction system was slowly cooled to room temperature, and a large amount of solid precipitated out, which was filtered and washed with water to obtain a yellow crude product. The crude product was recrystallized from 50% ethanol solution to obtain 2.87 g of a light yellow...
Embodiment 2
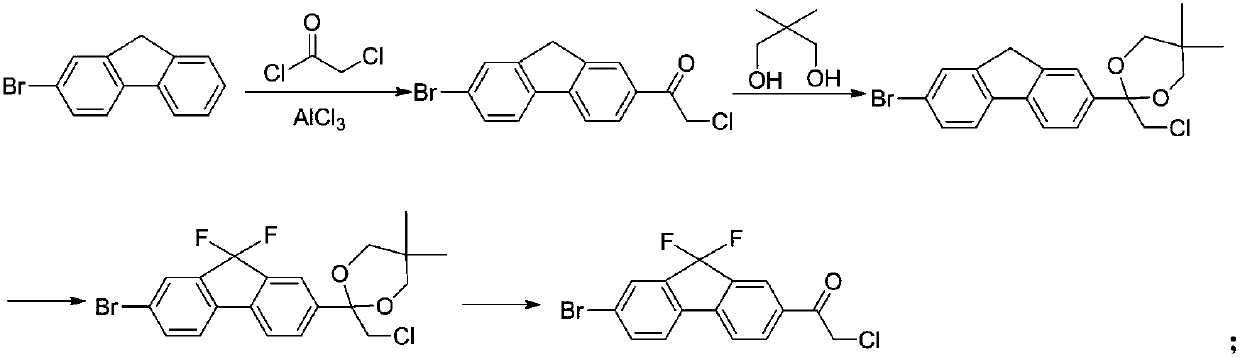
[0056] Synthesis (3) of embodiment 2,2-iodo-5-bromobenzoic acid methyl ester
[0057] Take 2-iodo-5-bromobenzoic acid (3.25g, 10mmol), dissolve it in 30ml of dichloromethane, add thionyl chloride (1.8g, 15mmol), 3 drops of DMF, stir at room temperature for 3h, after the reaction, reduce Remove the solvent and excess thionyl chloride under pressure, add 30ml of dichloromethane to the residue to dissolve, slowly add 20ml of methanol under ice bath, after the addition, the reaction system is warmed up to room temperature and continue to stir for 1h, after the reaction is over, under ice bath, Add 40ml of saturated aqueous sodium carbonate solution, stir for 5min, separate the liquids, wash the filtrate until neutral, dry the organic layer over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure. %. M.p.45-47°C.
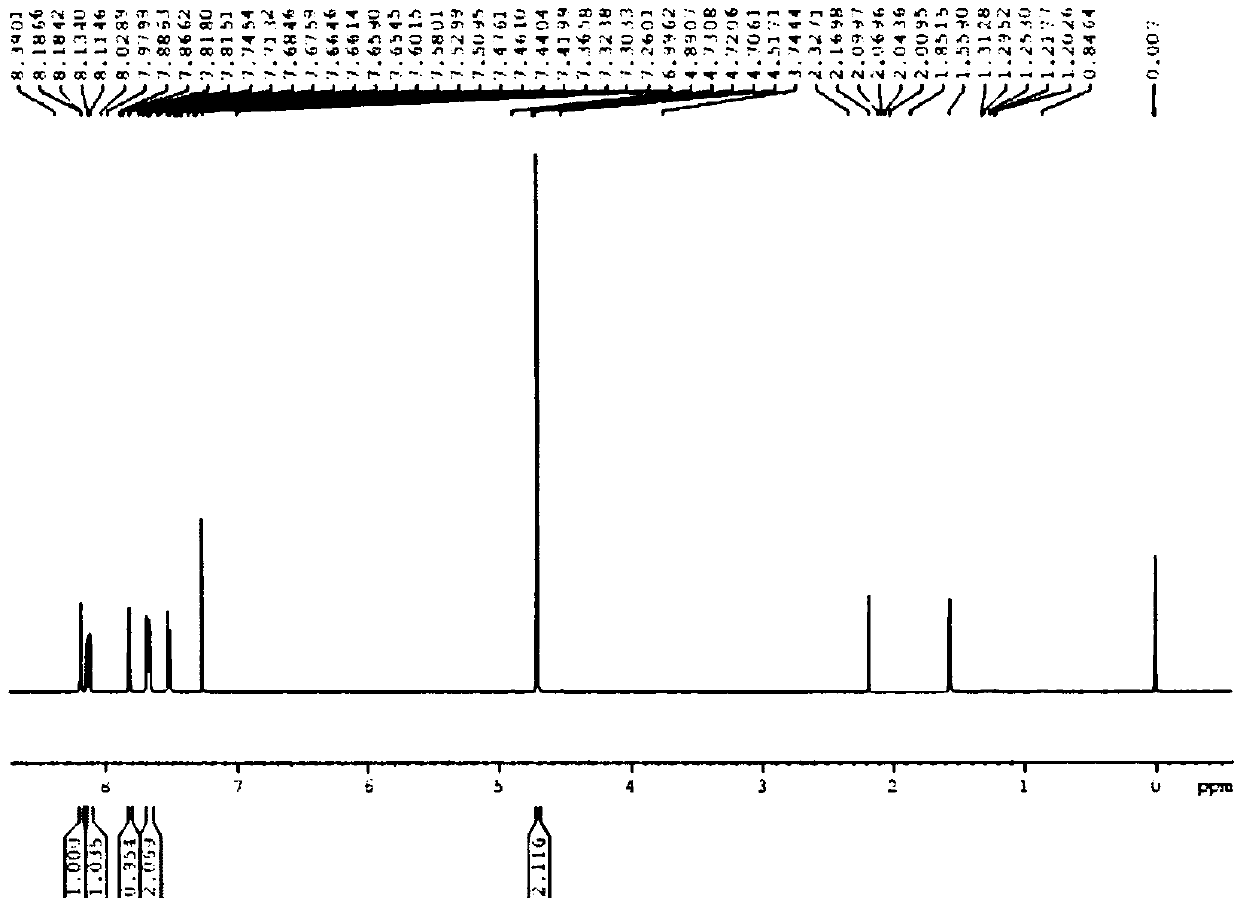
[0058] 1 HNMR (400MHz, CDCl 3 ): δ7.95(s, 1H), 7.86(d, J=8Hz, 1H), 7.31(d, J=8Hz, 1H), 3.96(s, 3H); 13 C NMR (100MHz, CDCl 3 )...
Embodiment 3
[0059] The synthesis (4) of embodiment 3,5-bromo-2-phenylbenzoic acid methyl ester
[0060] Take Pd(PPh3)2Cl2 (70mg, 0.1mmol), phenylboronic acid (1.28g, 10.5mmol), sodium carbonate (2.12g, 20mmol), water 30ml and THF 30ml. Under the protection of argon, the reaction system was stirred at room temperature for 5 min, and then methyl 4-bromo-2-iodobenzoate (3.39 g, 10 mmol) was added. Then the temperature of the reaction system was raised to 80° C. for 8 h. After the reaction, the reaction system was slowly cooled to room temperature, extracted with dichloromethane (50ml*3), the organic phases were combined, washed with water, the organic layer was dried over anhydrous sodium sulfate, filtered, the filtrate recovered the solvent under reduced pressure, and the residue was obtained by column chromatography. Color liquid 2.44g, yield 86%.
[0061] 1 H NMR (400MHz, CDCl 3 ): δ7.99(d, J=2.0Hz, 1H), 7.67(dd, J=8.4, 2.0Hz, 1H), 7.44–7.38(m, 3H), 7.31–7.28(m, 3H), 3.67( s,3H); 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com