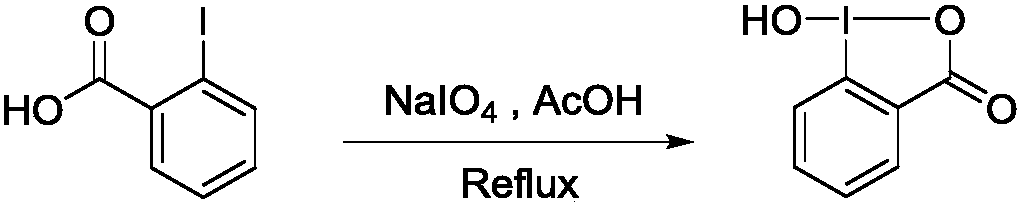
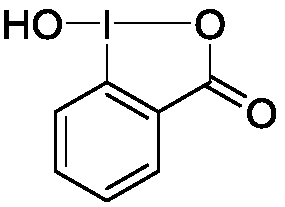
Preparation method of 1-hydroxyl-1,2-benziodoxol-3(1H)-one
A technology of phenyliodide and hydroxyl, which is applied in the field of synthesis of hypervalent iodine reagents, can solve problems such as difficult storage, expensive pentavalent iodine reagents, explosiveness, etc., and achieve the effects of high selectivity, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1、1
[0013] Implementation case 1, the preparation of 1-hydroxyl-1,2-phenyliodoacyl-3(1H)-ketone:
[0014]
[0015] Into a 250mL round bottom flask, add one No. 5 magneton, 10mmol o-iodobenzoic acid, 10mmol sodium periodate, 20mL glacial acetic acid, stir to completely dissolve the o-iodobenzoic acid, and reflux; the condenser tube flows from bottom to top at 25°C After condensing the water, carry out condensation and reflux, place the round-bottomed flask in a 120°C oil bath for magnetic stirring, and the magnetic stirring speed is 600 rpm / s. React for 4 hours in the dark; stop the reaction, add 50 mL of distilled water to dilute, and let stand to cool to room temperature, filter, collect the crude product, wash three times with 20 mL, 0 ℃ ice water and 20 mL of acetone, and dry in a dark place to obtain 2.45 g white 1-hydroxy-1,2-phenyliodo-3(1H)-one product, yield 93%.
[0016] Product H NMR 1 H NMR (400MHz, (CD3) 2 SO)δ8.06-7.95(m,3H); 7.86-7.84(m,1H); 7.73-7.69(m,1H). 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com