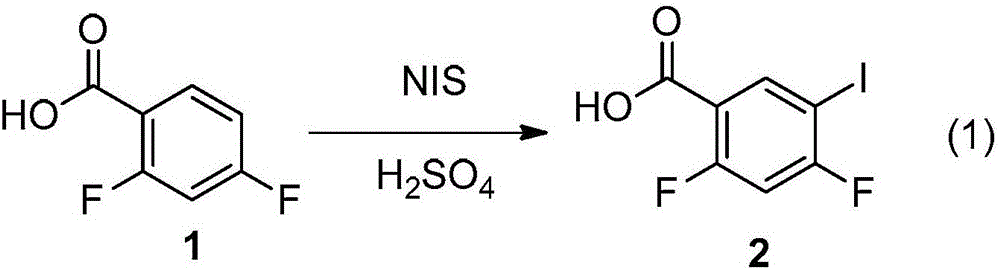
Preparation method of 2,4-difluoro-5-iodobenzoic acid
A technology of difluorobenzoic acid and iodobenzoic acid, which is applied in two fields, can solve problems such as oxidant waste, raw materials or difficult and expensive reactions, difficult iodine reactions, etc., and achieve simple post-processing, good yield, and good yield and the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
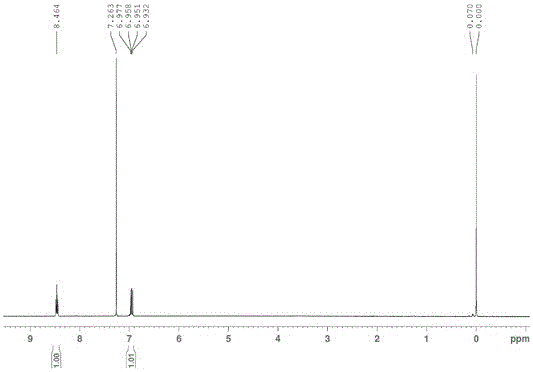
[0029] With glacial acetic acid 60ml, I 2 Add 5.33g (21mmol) into a 250ml three-necked flask, and slowly add 4.20g of sodium percarbonate (containing 31mmolH 2 o 2 ), raise the temperature to 35°C under stirring, and add 6.32g (40mmol) of 2,4-difluorobenzoic acid. Cool the reaction solution to 10°C, add 53.30ml of 98% H 2 SO 4 , after the addition, the temperature was raised to 45°C for 2.5 hours, and samples were applied to monitor the end of the reaction. After the reaction, cool the reaction solution, and use potassium iodide starch test paper to detect whether there are oxides in the reaction solution, and if so, use Na 2 SO 3 Aqueous solution (10g sodium sulfite dissolved in 500ml water, the configuration mass fraction is 2% solution) to remove peroxide. Then it was suction filtered and washed once with distilled water to obtain 2,4-difluoro-5-iodobenzoic acid with a yield of 76%. Melting point: 150-153°C.
[0030] Alternatives 1-11
[0031] The preparation metho...
Embodiment 2
[0040] Example 2: Amplified reaction
[0041] With glacial acetic acid 300ml, I 2 Add 26.7g (105mmol) into a 1L three-necked flask, and slowly add 21.0g of sodium percarbonate (SPC) (containing 155mmol H 2 o 2 ), raise the temperature to 35°C under stirring, and add 31.6g (200mmol) of 2,4-difluorobenzoic acid. Cool the reaction solution to 5°C, add dropwise 266.5ml of 98% H 2 SO 4 , after the addition, the temperature was raised to 50°C for 2 hours, and a sample was applied to monitor the end of the reaction. After the reaction, the reaction solution was cooled and washed with 2% Na 2 SO 3 aqueous solution to remove peroxides. Then it was suction filtered and washed to obtain 2,4-difluoro-5-iodobenzoic acid with a yield of 83%.
[0042] In conjunction with embodiment 1-2, and comparative example 1-2, it can be seen that:
[0043] 1. The present invention synthesizes 2,4-difluoro-5-iodobenzoic acid by the iodination reaction under acidic conditions, and iodine reacts ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com