Hydroboration reaction method of carbonic ester
A carbonate and hydroboration technology, applied in the field of hydroboration reaction, can solve the problem of high activity, and achieve the effects of easy synthesis, high yield and reduced catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
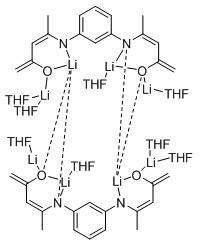
[0021] In the present invention, the preparation method of the above-mentioned catalyst lithium complex comprises the following steps, mixing the small molecule organolithium solution with the ligand solution, and then reacting to obtain the catalyst lithium complex; the chemical structural formula of the ligand is as follows:
[0022] .
[0023] In the present invention, in the small molecule organic lithium solution, the small molecule organic lithium includes n-butyllithium, and the solvent is an alkyl solvent, such as hexane; in the ligand solution, the solvent is an ether solvent, such as tetrahydrofuran.
[0024] In the present invention, the molar ratio of the small molecule organolithium to the ligand is 4:1, which has never been reported in the synthesis and application of the β-ketimine anion ligand.
[0025] specific:
[0026] m-Phenyl bridged β-ketoimine ligand (L ph h 2 )Synthesis
[0027]
[0028] Add 150 ml of absolute ethanol, 10.8 g of m-phenylenediam...
Embodiment 1
[0036] Embodiment one: [L ph’ Li 4 (THF) 4 ] 2 Catalytic Reduction of Ethylene Carbonate and Pinacol Borane
[0037] Under an inert gas atmosphere, 5.84 mg (0.005 mmol) of the catalyst was added to the reaction flask after dehydration and deoxygenation treatment, ethylene carbonate (33.3 μL, 0.5 mmol), pinacol borane (239.4 μL ,1.65 mmol), THF (200 μL), at 60 o After reacting in C for 120 min, use mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stir evenly, draw a drop into the NMR tube with a dropper, and add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 3.90 (s, 4H, OCH 2 ), 1.21 (s, 24H, OBpin).
Embodiment 2
[0042] Embodiment two: [L ph’ Li 4 (THF) 4}] 2 Catalytic Reduction of Propylene Carbonate and Pinacol Borane
[0043] Under an inert gas atmosphere, 5.84 mg of the catalyst was added to the dehydrated and deoxygenated reaction flask, and propylene carbonate (42.4 μL, 0.5 mmol) and pinacol borane (239.4 μL, 1.65 mmol) were sequentially added with a pipette gun. , THF (200 μL), at 60 o After reacting in C for 120 min, use mesitylene (69.6 μL, 0.5 mmol) as the internal standard, stir evenly, draw a drop into the NMR tube with a dropper, and add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 4.28-4.20 (m, 1H, CH 3 CH), 3.71 (d, J = 5.6 Hz, 2H, OCH 2 ), 1.21 (s, 12H, OBpin), 1.20 (s, 12H, OBpin).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com