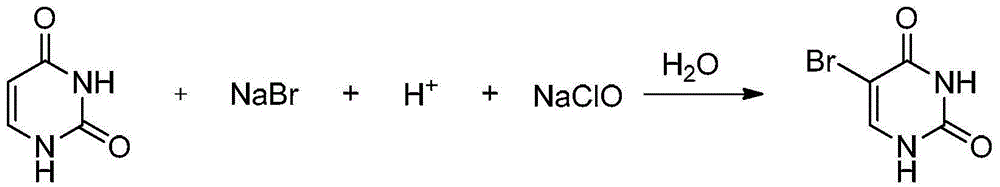Preparation method for 5-bromouracil
A technology for bromouracil and uracil, applied in the field of drug synthesis, can solve the problems of large environmental pollution, low uracil yield, troublesome post-processing and the like, and achieve the effects of simple post-processing, high yield and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 45ml of hydrochloric acid (6mol / L), 10g of uracil (89mmol) and 18.4g of NaBr (178mmol) into a 250mL three-neck flask, heat and stir at 50°C for half an hour, then cool to room temperature, and stir rapidly Add 54mL of NaClO (2mol / L) aqueous solution dropwise, react at room temperature for 3 hours after the addition is complete, and monitor the reaction end point with a sample. After the reaction, use starch potassium iodide test paper to detect whether there is NaClO in the reaction solution. Sodium sulfosulfate removes excess NaClO. Then adjust the pH value to about 8-9 with 6mol / L NaOH to obtain a white solid, filter it with suction and wash once with ice ethanol to obtain 15.6 g of 5-bromouracil with a yield of 91.5%.
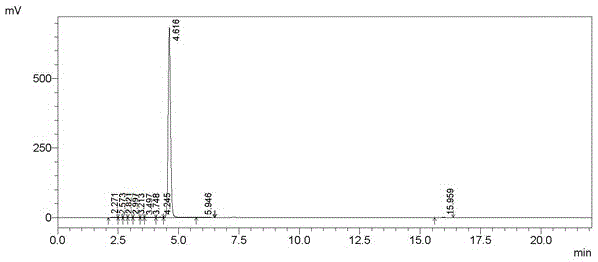
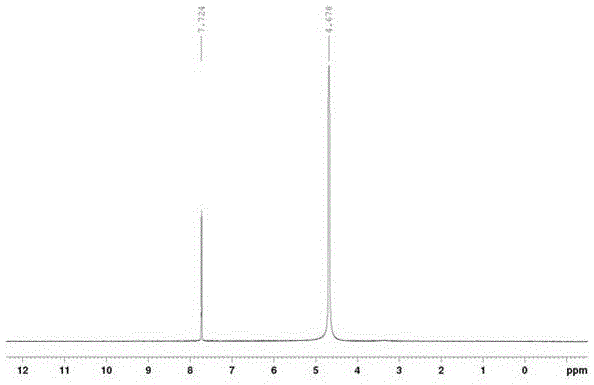
[0035] Product confirmation: the liquid chromatogram and NMR spectrum of the prepared product are as follows: figure 1 , figure 2 shown.
[0036] Replacement examples 1-1 to 1-7:
[0037] The preparation method is the same as in Example 1, th...
Embodiment 2
[0043] Add 12ml of acetic acid, 10g of uracil (89mmol) and 18.4g of NaBr (178mmol) into a 250mL three-neck flask, heat and stir at room temperature for half an hour, then add dropwise 54mL of NaClO (2mol / L) aqueous solution under rapid stirring, After the addition, react at room temperature for 3 hours, and monitor the end point of the reaction with a sample. After the reaction, use starch potassium iodide test paper to detect whether there is NaClO in the reaction solution. If so, remove excess NaClO with saturated sodium thiosulfate. Then adjust the pH value to about 8-9 with NaOH to obtain a white solid, which was filtered by suction and washed once with ice ethanol to obtain 15.1 g of 5-bromouracil with a yield of 88.6%.
Embodiment 3
[0045] Mix 10g of uracil (89mmol) and 5mL of water in a 250mL three-necked flask, add 21ml of concentrated sulfuric acid dropwise under stirring, stir for 5‐10 minutes after the drop is complete, then add 18.4g of NaBr (178mmol), and then stir rapidly Add 54mL of NaClO (2mol / L) aqueous solution dropwise, react at room temperature for 3 hours after the addition is complete, and monitor the reaction end point with a sample. After the reaction, use starch potassium iodide test paper to detect whether there is NaClO in the reaction solution. Sodium sulfosulfate removes excess NaClO. Then adjust the pH value to about 8-9 with 6M NaOH to obtain a white solid, filter it with suction, and wash once with ice ethanol to obtain 16.2 g of 5-bromouracil with a yield of 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com