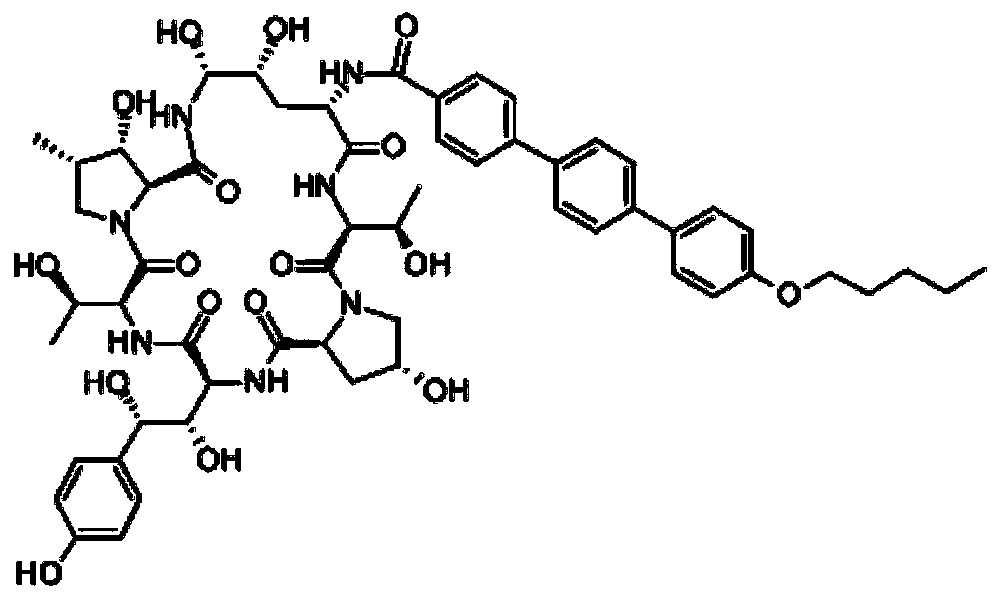
Preparation method of anidulafungin intermediate
A technology of anidungin and intermediates, applied in the field of drug synthesis, can solve problems such as not being suitable for industrial production, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
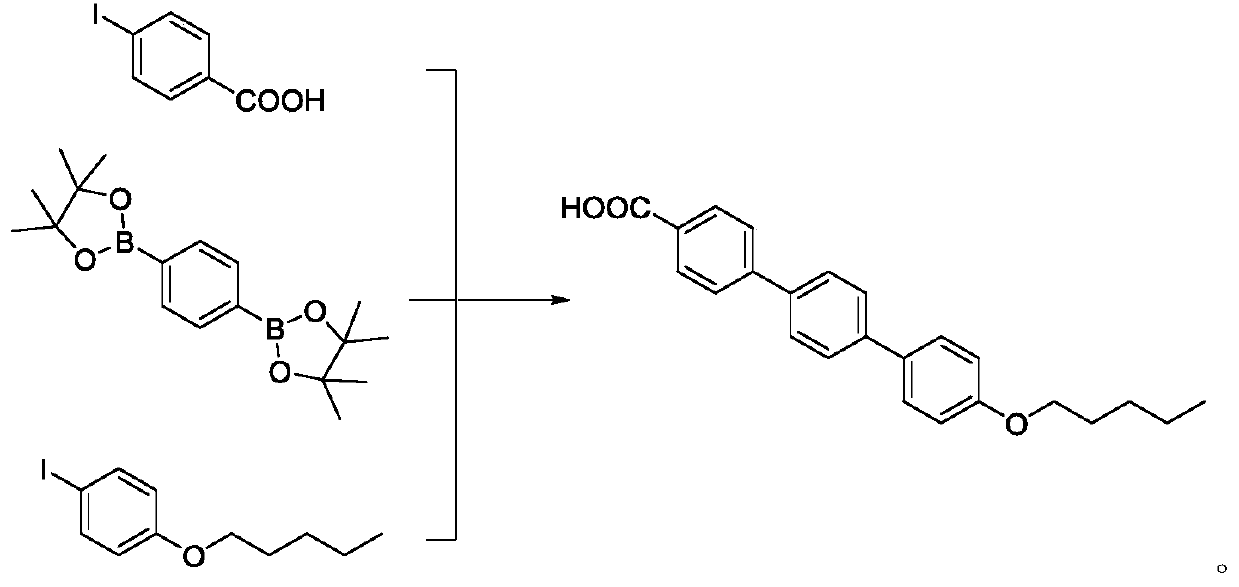
[0023] At room temperature, add potassium carbonate (190g), water (750mL) and DMF (750mL) to the reaction flask, and add 4-iodobenzoic acid (53g), 1,4-benzenediboronic acid pinacol ester indole under stirring condition (93g) under nitrogen protection, add tetrakis (triphenylphosphine) palladium (2.7g), be heated to 60 ℃ of reaction, after reacting for 3 hours, slowly add 4-n-pentyloxy iodobenzene (62g) in the reaction system DMF (130mL) solution was added, and the reaction was continued for 4 hours. Cool the reaction solution to 5-10°C, adjust the pH of the system to 4 with 0.5M dilute hydrochloric acid under stirring, continue stirring for 1 hour, filter, wash the filter cake with water, and then wash the filter cake with alcohol, and dry to obtain p-amyl Terbenzoic acid (82 g), HPLC purity 99.9%.
Embodiment 2
[0025] At room temperature, add sodium carbonate (100g), water (500mL) and DMF (800mL) into the reaction flask, add 4-iodobenzoic acid (39g), 1,4-benzenediboronic acid pinacol ester indole under stirring condition (59g) Under the protection of nitrogen, add [1,1'-bis(diphenylphosphino)ferrocene]palladium dioxide (2.1g), heat to 60°C for reaction, after 2.5 hours of reaction, slowly pour into the reaction system A solution of 4-n-pentyloxyiodobenzene (45 g) in DMF (100 mL) was added, and after the addition was complete, the reaction was continued for 3 hours. The reaction solution was cooled to 5-10°C, and the pH value of the system was adjusted to 4 by using 0.5M dilute hydrochloric acid under stirring conditions, and continued to stir for 0.5 hours, filtered, washed the filter cake with water and ethanol, and dried to obtain p-pentyleneterphenylcarboxylic acid (55g ), HPLC purity 99.8%.
Embodiment 3
[0027] At room temperature, add sodium carbonate (1000g), water (4L) and DMF (6L) to the reaction flask, add 4-iodobenzoic acid (390g), 1,4-benzenediboronic acid pinacol ester indole under stirring conditions (590g) under the protection of nitrogen, add [1,1'-bis(diphenylphosphino)ferrocene]palladium dioxide (21g), heat to 60°C for reaction, after reacting for 3 hours, slowly add DMF (1 L) solution of 4-n-pentyloxyiodobenzene (450 g) was added, and the reaction was continued for 6 hours. The reaction solution was cooled to 5-10°C, and the pH value of the system was adjusted to 4 by using 0.5M dilute hydrochloric acid under the stirring condition, and the stirring was continued for 2 hours, filtered, and the filter cake was washed with water and ethanol, and dried to obtain p-pentyleneterphenylcarboxylic acid (550g ), HPLC purity 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com