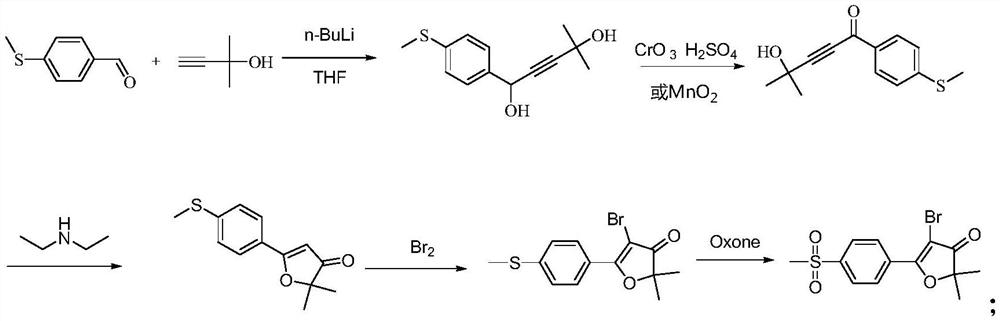
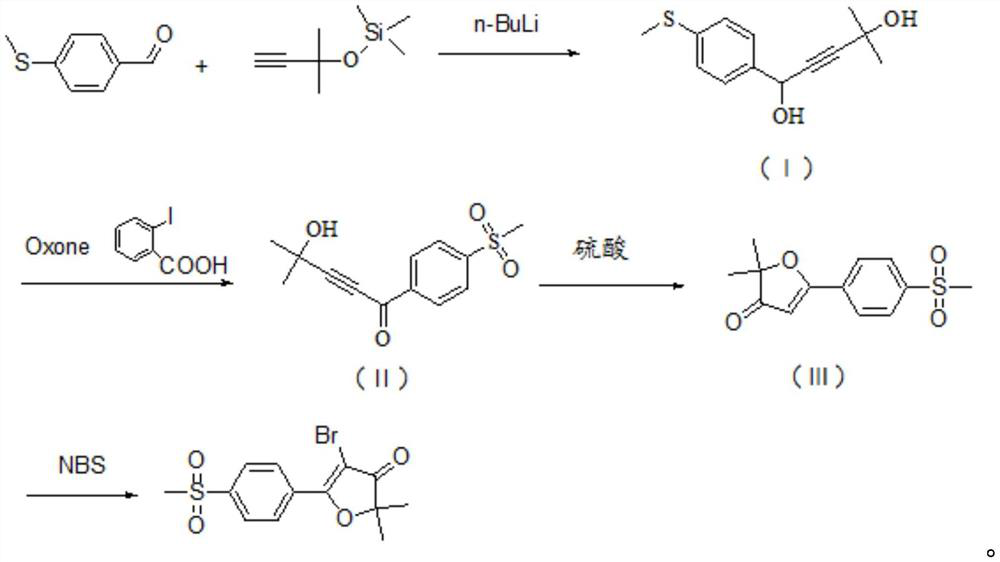
Synthesis of 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2h)-one
The technology of a methanesulfonyl phenyl group and a synthesis method, which is applied in the field of organic synthesis, can solve the problems of being unsuitable for industrial production, the residual fluctuation of reaction raw materials is large, and achieve the effects of easy industrial production, avoiding nitrogen-containing waste water and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The invention provides a method for synthesizing 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2H)-one, comprising the following steps:
[0036] S1: 4-methylthiobenzaldehyde and [(1,1-dimethyl-2-propynyl)oxy]trimethylsilane undergo nucleophilic addition reaction under the action of n-butyllithium to generate A compound of formula (I);
[0037] S2: The compound of formula (I) of S1 reacts with Oxone and o-iodobenzoic acid to generate the compound of formula (II);
[0038] S3: S2 The compound of formula (II) undergoes a ring-closing reaction under the catalysis of sulfuric acid to generate the compound of formula (III);
[0039] S4: S3 formula (Ⅲ) compound reacts with NBS to produce 5-bromo-2,2-dimethyl-5-[4-(methylsulfonyl)phenyl]furan-3(2H)-one ;
[0040] Its synthetic route is as follows:
[0041]
Embodiment 1
[0043] A method for synthesizing 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2H)-one, specifically comprising the following steps:
[0044] Step 1, the synthesis of formula (I) compound:
[0045]Under the protection of nitrogen (500mL / min), add [(1,1-dimethyl-2-propynyl) Oxygen]trimethylsilane 185.60g, tetrahydrofuran 1L, after stirring for 10min, slowly cool down to -90°C, start to add 594.5mL of n-butyllithium dropwise, after stirring at -90°C for 1h, add dropwise to the reaction system to configure 124.81g of 4-methylthiobenzaldehyde and 124mL of tetrahydrofuran were added dropwise, and the reaction was stopped after stirring at -90°C for 2h, and the reaction solution was slowly poured into aqueous hydrochloric acid (concentrated hydrochloric acid: 187.2mL, water: 324.5mL ), stirred for 1 hour, then separated the liquid, collected the organic phase, washed the organic phase with water until neutral, separated the liquid, concentrated the organic phase under norm...
Embodiment 2
[0053] A method for synthesizing 5-bromo-2,2-dimethyl-5-(4-methylsulfonylphenyl)furan-3(2H)-one, specifically comprising the following steps:
[0054] Step 1, the synthesis of formula (I) compound:
[0055] Under the protection of nitrogen (500mL / min), add [(1,1-dimethyl-2-propynyl) Oxygen]trimethylsilane 185.60g, tetrahydrofuran 876mL, after stirring for 10min, slowly cool down to -70°C, start to add 594.5mL of n-butyllithium dropwise, after stirring at -70°C for 1h, add dropwise to the reaction system to configure 124.81g of 4-methylthiobenzaldehyde and 124mL of tetrahydrofuran were added dropwise, and the reaction was stopped after stirring at -70°C for 2h, and the reaction solution was slowly poured into aqueous hydrochloric acid (concentrated hydrochloric acid: 187.2mL, water: 324.5mL ), stirred for 1 hour, then separated the liquid, collected the organic phase, washed the organic phase with water until neutral, separated the liquid, concentrated the organic phase under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com