A synthetic method of 2-chloro-5-iodobenzoic acid
A technology of iodobenzoic acid and synthesis method, which is applied in chemical instruments and methods, preparation of carboxylate, preparation of oxygen-containing compounds, etc., can solve the problems of complex synthesis method, low yield, troublesome operation, etc., and achieves simple process and high production efficiency. Process safety and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
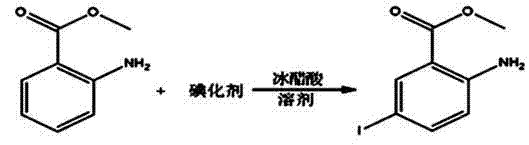
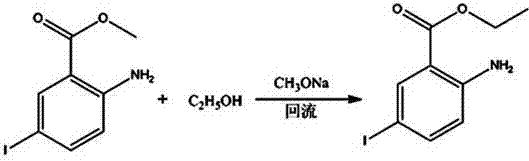
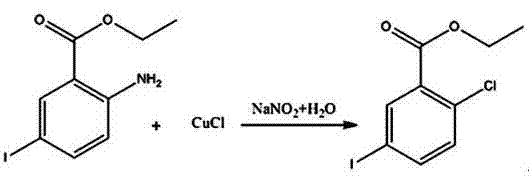
[0027] A kind of synthetic method of 2-chloro-5-iodobenzoic acid:
[0028] 1. Iodine, substitution
[0029] Determine the mass ratio of raw materials and reagents as methyl anthranilate: potassium iodate: iodide: dichloromethane: water: sodium sulfite: ethanol: sodium methoxide methanol solution: acetic acid: water = 1: 0.4~0.5: 0.8~ 1:2~5:1.5~3:0.008~0.01:3~5:0.25~0.4:0.02~0.05:0.08~0.1
[0030] Add 588ml of water and 242.4g of potassium iodide into a 2L three-necked flask and stir to dissolve. Add 139.1g of potassium iodate, stir to dissolve, add 300g of methyl anthranilate, then add 99.3ml of dichloromethane, add 191.8g of glacial acetic acid dropwise through the dropping funnel, finish adding in about 1.5 hours, and the temperature rises to 50 ℃, stirred for 30 minutes after the addition, the temperature rose to 55 ℃ and kept for 2 hours, cooled to 35 ℃, added 397.4ml of dichloromethane, stirred for 15 minutes, then added 2.8g of solid sodium sulfite in batches, cooled t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com