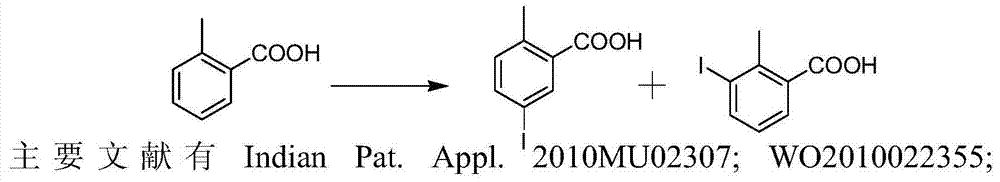
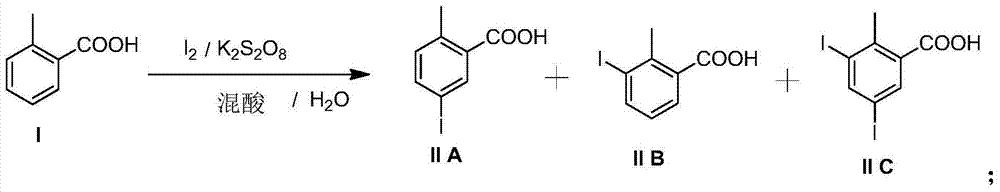
Preparation and recovery method of 2-methyl-5-iodobenzoic acid
A technology of o-toluic acid and iodobenzoic acid is applied in the field of preparation and recovery of 2-methyl-5-iodobenzoic acid, and can solve problems such as being unsuitable for industrial production, long reaction process, complicated handling and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of 2-methyl-5-iodobenzoic acid
[0027] 500ml reaction bottle, nitrogen protection, add acetic acid (200g), water (20g), concentrated sulfuric acid (20g) at room temperature, stir and mix well, then add o-toluic acid (49g, 0.36mol), potassium persulfate (50g) in sequence , finally add iodine (38g, 0.15mol), the temperature of the solution rises slightly, after stirring for 30 minutes, slowly raise the temperature to 50°C, and keep it for 1 hour; then slowly raise the temperature to 70°C, keep it for 2 hours; -4 hours, the color of iodine in the reaction solution gradually disappeared, and the reaction solution was pale yellow. Reaction liquid sampling HPLC analysis (o-toluic acid ~ 10%; 2-methyl-5-iodobenzoic acid ~ 75%; 2-methyl-3-iodobenzoic acid ~ 15%; 2-methyl-3, 5-Diiodobenzoic acid <1%).
[0028] The reaction was stopped, and the reaction solution was slowly cooled to 15-20°C, filtered, washed, and dried to obtain ~55g of crude product (yield 70%, calc...
Embodiment 2
[0035] Synthesis of 2-methyl-5-iodobenzoic acid
[0036] 500ml reaction bottle, under nitrogen protection, add propionic acid (200g) and water (20g) at room temperature and mix well, then add concentrated sulfuric acid (20g), o-toluic acid (49g, 0.36mol), potassium persulfate (50g) in sequence , finally add iodine (38g, 0.15mol), the temperature of the solution rises slightly, after stirring for 30 minutes, slowly raise the temperature to 50°C, and keep it for 1 hour; then slowly raise the temperature to 70°C, keep it for 2 hours; -4 hours, the color of iodine in the reaction solution gradually disappeared, and the reaction solution was light yellow. Reaction liquid sampling HPLC analysis (o-methylbenzoic acid ~ 8%; 2-methyl-5-iodobenzoic acid ~ 80%; 2-methyl-3-iodobenzoic acid ~ 11%; 2-methyl-3, 5-Diiodobenzoic acid <1%).
[0037] The reaction was stopped, and the reaction solution was slowly cooled to 15-20°C, filtered, washed, and dried to obtain ~58.9 g of crude product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com