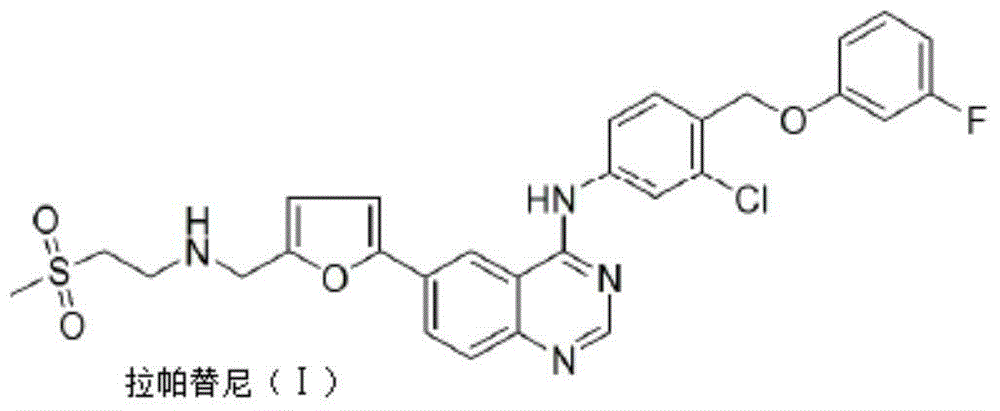
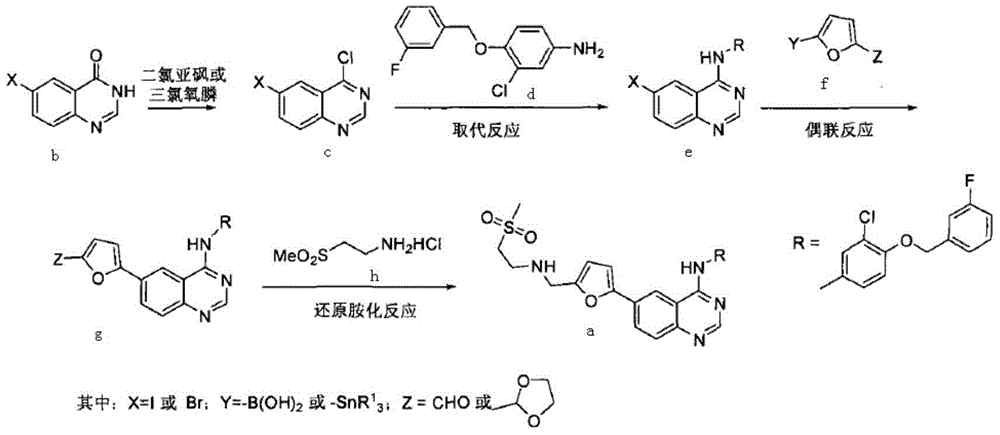
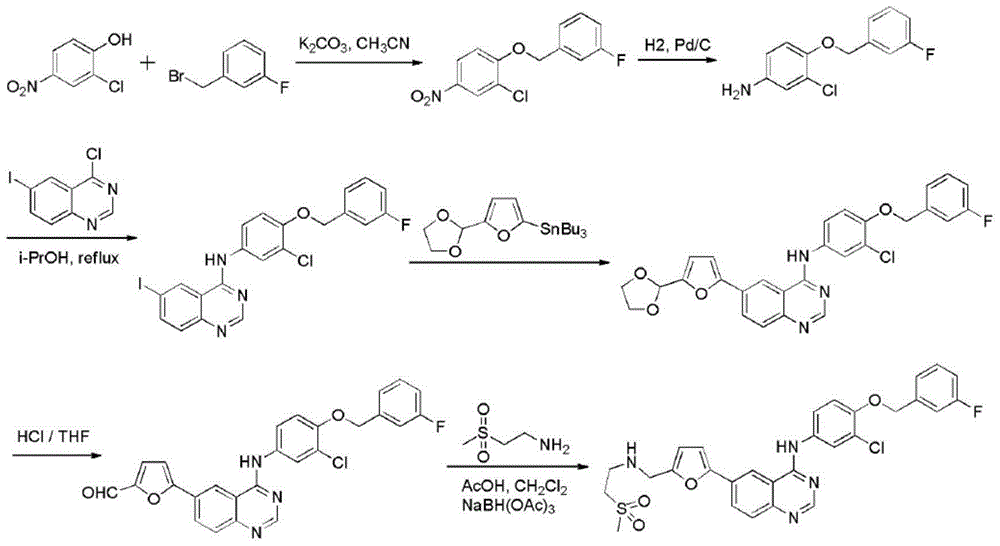
Lapatinib preparation method
A lapatinib and compound technology, which is applied in the field of drug synthesis, can solve the problems of stannyl furan compound pollution, reduced yield of coupling products, unstable boronic acid group, etc., and achieves reduction of production cost, reduction of reaction steps, and increase of solubility. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 6-iodo-3,4-dihydroquinazolin-4-one (Ⅲ)
[0037] 2-Amino-5-iodobenzoic acid (II) (6.8g, 0.05mol), mixed with 20ml of formamide, heated at 180°C, stirred for 12h, cooled, poured into ice water, filtered, washed with water to obtain 6- Iodine-3,4-dihydroquinazolin-4-one (Ⅲ) needle crystal 12.6g, yield 92.6%, HPLC purity 99.83%.
Embodiment 2
[0039] Preparation of 6-iodo-3,4-dihydroquinazolin-4-one (Ⅲ)
[0040] 2-Amino-5-iodobenzoic acid (II) (6.8g, 0.05mol), mixed with 15ml of formamidine acetate, heated at 160°C, stirred for 12h, cooled, poured into ice water, filtered, washed with water to obtain 6 -Iodine-3,4-dihydroquinazolin-4-one (Ⅲ) needle crystal 12.4 g, yield 90.9%, HPLC purity 99.56%.
Embodiment 3
[0042] Preparation of 6-iodo-3,4-dihydroquinazolin-4-one (Ⅲ)
[0043] 2-Amino-5-iodobenzoic acid (II) (6.8g, 0.05mol), mixed with 20ml of formamide, heated at 100°C, stirred for 15h, cooled, poured into ice water, filtered, washed with water to obtain 6- Iodine-3,4-dihydroquinazolin-4-one (Ⅲ) needle crystal 11.7g, yield 86.7%, HPLC purity 99.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com