Preparation method of pemetrexed disodium
A technology of pemetrexed disodium and dihydrogen, which is applied in the field of new preparation of pemetrexed disodium, can solve the problems of many steps and increased product cost, and achieve the effects of avoiding oxidation, high product purity and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
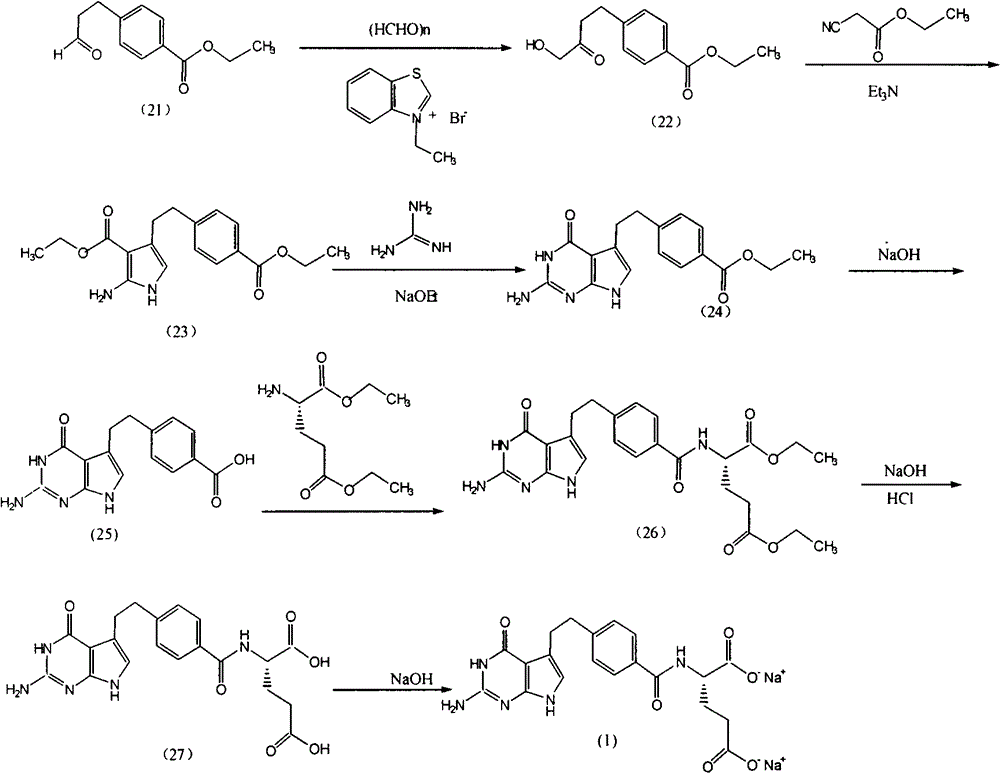
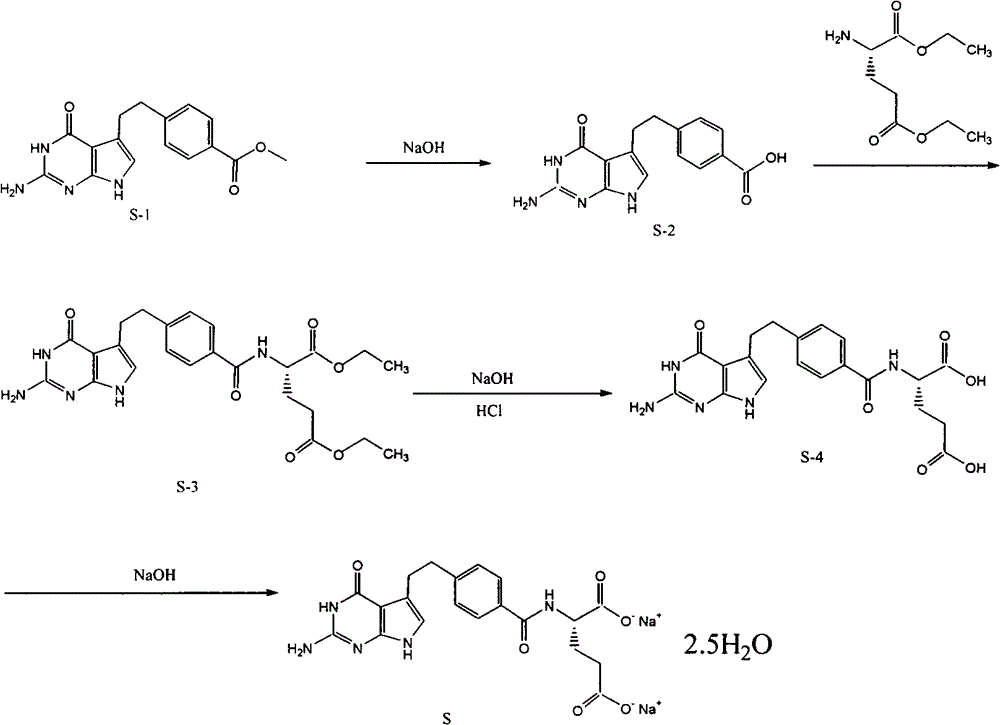
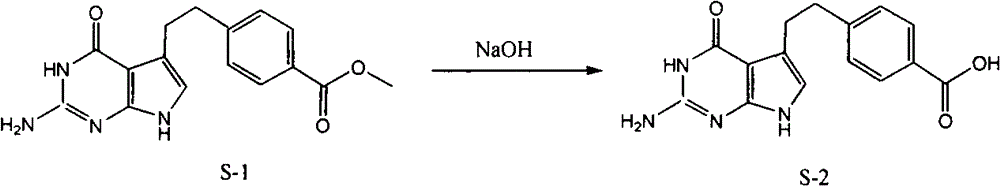
[0037] (1). Synthesis of 4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid ( S-2)
[0038] Reaction formula:
[0039]
[0040] Feeding:
[0041] 4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid methyl ester (S-1 ) 58.4g
[0042] 2mol / L sodium hydroxide 200ml
[0043] Ethanol 200ml
[0044]Add 58.4g of S-1 and 200ml of 2mol / l sodium hydroxide into the three-neck flask, stir and react at 45°C for 3 hours, TLC detects that the raw material point basically disappears as the end of the reaction (developing agent: ethyl acetate:cyclohexane=4:1) . Add 200ml of ethanol, adjust the pH of the reaction solution to 4 with 5mol / L hydrochloric acid under water cooling, and precipitate a yellow solid, filter it, wash it with 50% ethanol, and dry it under vacuum at 50°C for 5 hours to obtain 40.6g of a light yellow solid 4-[2- (2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid (S-2), yiel...
Embodiment 2
[0068] In Example 1, except that the ratio of ethanol and dimethyl sulfoxide used in steps 4 and 5 was adjusted to 10:1.9, the rest were the same as in Example 1 to obtain 22.0 g of pemetrexed disodium two and a half The crystallization of the hydrate has a yield of 80.9%, and the HPLC purity of the product is 99.6%.
Embodiment 3
[0070] In Example 1, except that the ratio of ethanol and dimethyl sulfoxide used in steps 4 and 5 was adjusted to 10:2.5, the rest were the same as in Example 1 to obtain 22.1 g of pemetrexed disodium two and a half The crystallization of the hydrate has a yield of 81.2%, and the HPLC purity of the product is 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com