Radiosynthetic method of subglutamic acid pet imaging agent
A technology of radiosynthesis and glutamic acid, applied in the direction of organic chemical methods, chemical instruments and methods, and preparation of carboxylic acid amides, can solve the problems of long time for radiosynthesis, difficulty in automatic production, and low radiochemical yield, and achieve a solution Effects of Automated Production Difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
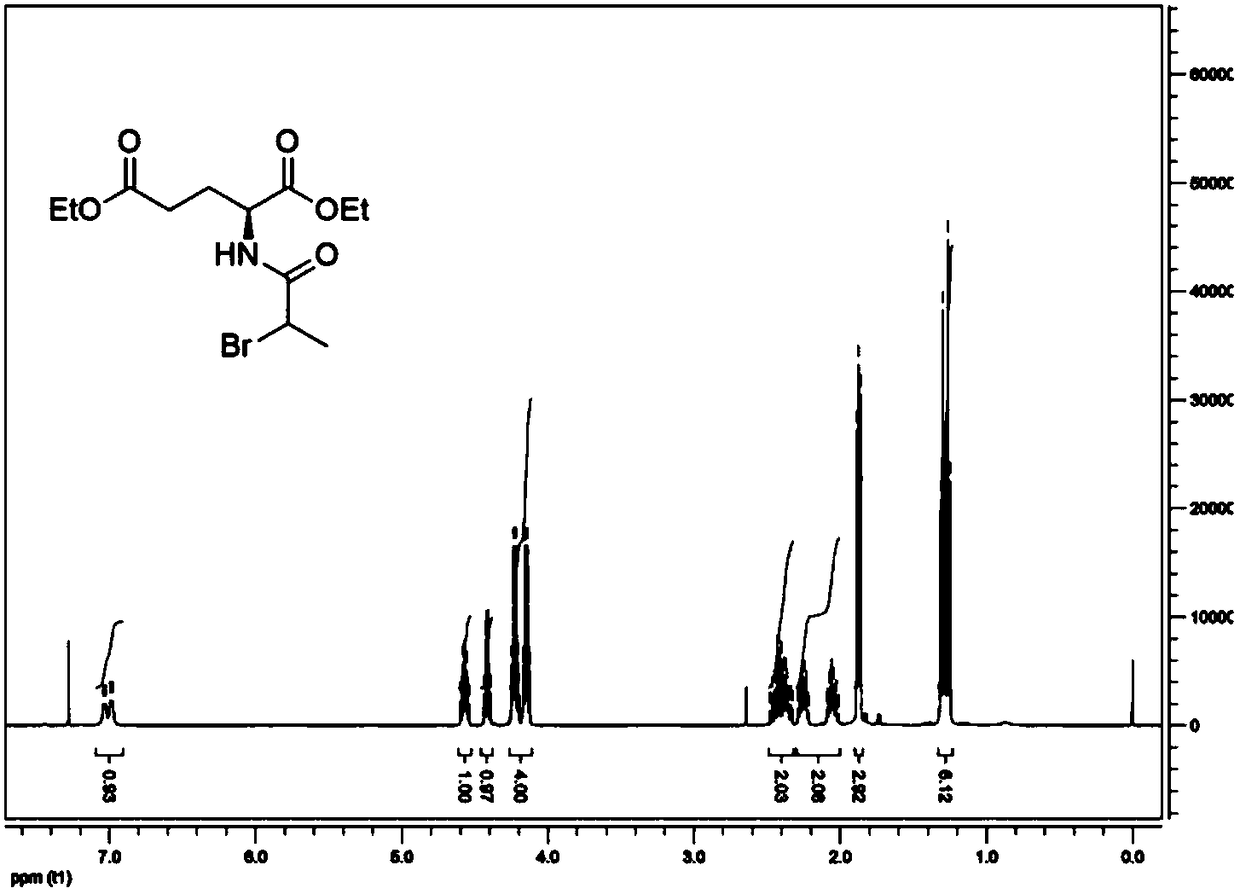
[0029] The preparation of embodiment 1 precursor and standard
[0030] 1.1 Precursor (N-2-bromopropionyl)-L-α-diethyl glutamate and standard (N-2- 19 F-fluoropropionyl)-L-α-glutamic acid ( 19 Preparation of F-NFPGlu). Its synthetic routes are shown in Reaction Schemes 4 and 5, respectively.
[0031] 2-Bromopropionyl bromide (3.24g, 15.00mmol) was added to L-diethyl glutamate hydrochloride (3.17g, 15.00mmol) and N,N-diisopropyl Diethylamine (4.07 g, 31.50 mmol) in anhydrous dichloromethane (50 mL) was reacted overnight at room temperature. After the reaction, dilute with water (100mL), extract three times with dichloromethane, 50ml each time (3*50mL), combine organic phase, 10% citric acid aqueous solution (100mL), water (100mL) and saturated sodium chloride aqueous solution (100mL ) were washed once each, and then washed with anhydrous Na 2 SO 4 Drying, filtration, and concentration to remove the organic solvent gave white solid (N-2-bromopropionyl)-L-α-glutamic acid die...
Embodiment 2
[0041] Synthesis of embodiment 2 hypoglutamic acid PET imaging agent
[0042] 2.1 Synthesis method of subglutamic acid PET imaging agent. The synthetic routes are shown in Reaction Formulas 1, 2, and 3, respectively.
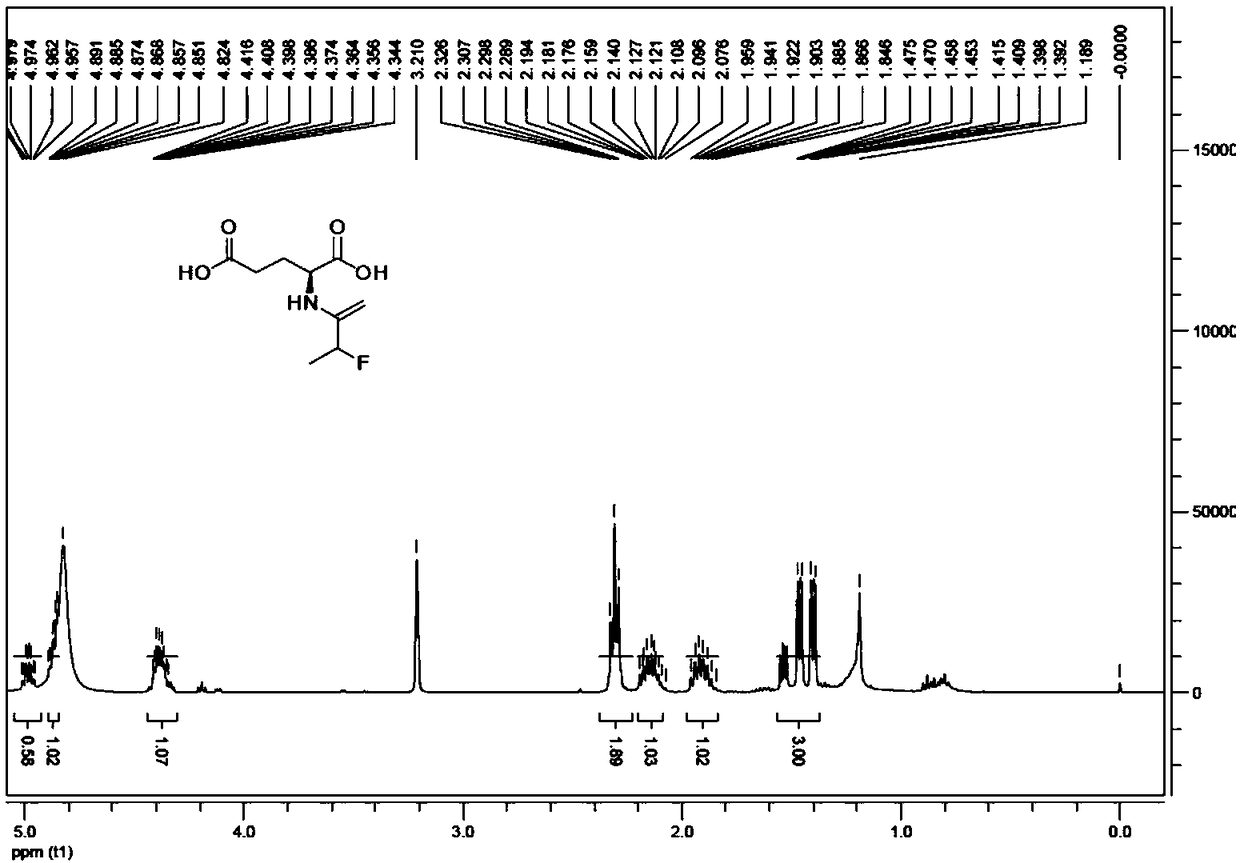
[0043] (N-2- 18 F-fluoropropionyl)-L-α-glutamic acid ( 18 F-NFPGlu), using (N-2-bromo-propionyl)-L-α-diethyl glutamate as the precursor raw material, through the two-step reaction of nucleophilic fluorination and "in-column hydrolysis", it can realize its radiosynthesis. 1) Fluorination reaction, (N-2-bromo-propionyl)-L-α-diethyl glutamate, in amino polyether Kryptofix2.2.2 (K 2.2.2 ) under the catalysis and [K / K2.2.2] +18 f - A nucleophilic substitution reaction occurs, producing 18 F labeled intermediate (N-2- 18 F-fluoropropionyl)-L-α-glutamic acid diethyl ester. After diluting with water, the mixed solution was passed through a Sep-Pak Al 2 o 3 A series column of N Plus column and two Sep-Pakplus C18 cartridges, radioactive intermediates are captu...
Embodiment 3
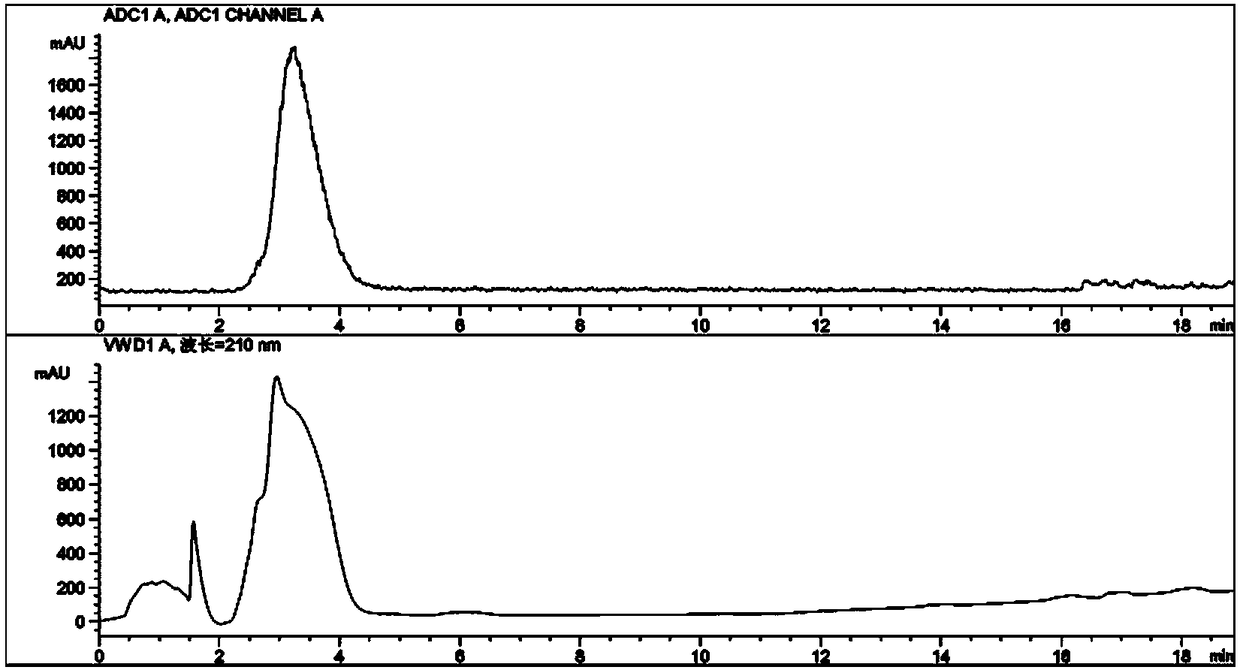
[0049] Example 3 Determination of product purity.
[0050] Determination by radioactive high performance liquid chromatography (HPLC) 18 F-NFPGlu, 18 F-DFPGlu or 18 Radiochemical purity of F-NFPBGlu injection. Standards with defined structure 19 F-NFPGlu, 19 F-DFPGlu or 19 F-NFPBGlu, respectively with the corresponding injection 18 F-NFPGlu, 18 F-DFPGlu or 18 The F-NFPBGlu injection is injected into the HPLC together to determine whether its retention time is consistent, so as to confirm the accuracy of the prepared injection. It has been determined that its radiochemical purity is greater than 90%, and the typical test results are shown in Figure 6 and Figure 7 . HPLC analysis conditions: analytical column is ZORBAX Eclipse XDB-C18 column, mobile phase is 0.1% TFA acetonitrile solution: 0.1% TFA aqueous solution, line gradient elution: at 0min, acetonitrile solution containing 0.1% TFA / 0.1% TFA Aqueous solution: 2 / 98; when gradually increasing to 8 minutes, 0.1%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com