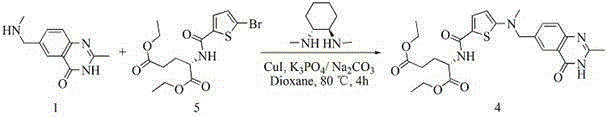Synthesis methods of raltitrexed
A compound and aqueous solution technology, applied in the field of anti-tumor drug synthesis, can solve the problems of complex and lengthy synthesis steps, unobtainable raw materials, large environmental pollution, etc., to solve the problems of toxicity and safety, avoid post-processing difficulties, and the total yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The experimental condition screening of embodiment one preparation method of the present invention
[0054] Now mainly illustrate the synthesis process research process of the intermediate compound 1 of the present invention.
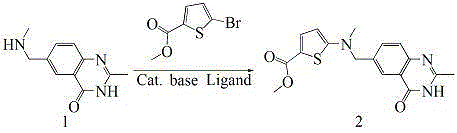
[0055] (1), preparation of 6-((methylamino)methyl)-3,4-dihydro-2-methyl-4-oxo-6-quinazoline (compound 1)
[0056] .
[0057] The reaction step is an amination reaction, and the raw material 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline reacts with methylamine to obtain 6-((methylamino) Methyl)-3,4-dihydro-2-methyl-4-oxo-6-quinazoline (Compound 1). In the research, the solvent was first screened. We used small molecule alcohol as the solvent. The experimental results found that in the alcohol solvent, the reaction occurred rapidly. In 10 to 20 minutes, TLC detected the raw material 6-bromomethyl-3,4-di The reaction of hydrogen-2-methyl-4-oxo-6-quinazoline is complete, but there are many impurity spots and the impurity situation is co...
Embodiment 2
[0094] Embodiment two adopts scheme one method to prepare raltitrexed
[0095] (1) Preparation of 6-((methylamino)methyl)-3,4-dihydro-2-methyl-4-oxo-6-quinazoline (compound 1)
[0096] .
[0097] Slowly add 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline (m =25.3g, n=0.1mol), the temperature is controlled at -10°C~-5°C, after the dropwise addition is completed, the temperature is gradually raised to room temperature, and the stirring is continued to confirm that the reaction is complete (TLC detection). Add sodium bicarbonate solution to the reaction solution, adjust the pH to alkaline (pH>7), extract with dichloromethane, wash the organic phase with saturated aqueous sodium chloride solution, remove the solvent by rotary evaporation of the organic phase, and then add 500 mL of water for beating , stirred for 30 minutes, filtered, the filter cake was washed with water (3×100mL), drained and dried to obtain an off-white solid (14.1g, 69.4%).
[0098] 1 HNMR (400MHz...
Embodiment 3
[0115] Embodiment three adopts scheme two method to prepare raltitrexed
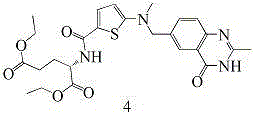
[0116] (1), the preparation of N-(5-bromothien-2-yl) diethyl glutamate (compound 5)
[0117] .
[0118] Add 200 mL of DMF, 10.15 g of L-diethylglutamate (1 eq), 11.39 g of 5-bromothiophene-2-carboxylic acid (1.1 eq), 10.13 g of HOBT (1.5 eq), 19.35 g of N,N-diisopropyl Ethylamine (3eq), 14.40gEDCI (1.5eq), under nitrogen protection conditions, stirred at room temperature for reaction, after the reaction was complete, added 500mL ethyl acetate for extraction, the mixture was washed 5 times with saturated saline, and the organic phase was washed with anhydrous Dry over sodium sulfate, filter, evaporate the solvent, and dry to obtain 16.50 g of diethyl N-(5-bromothien-2-yl)glutamate, with a yield of 84.2%.
[0119] 1 HNMR (CDCl 3 )δ7.30-7.27(m,1H),7.05-7.03(m,1H),7.01-6.98(m,1H),4.72-4.68(m,1H),4.24-4.22(m,2H),4.13- 4.10(m,2H),2.52-2.37(m,2H),2.31-2.25(m,1H),2.17-2.13(m,1H),1.31-1.29(m,3H),1.25-1.22(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com