Epoxidized soybean oil itaconate as well as preparation method and application thereof
A technology of oil itaconate and epoxidized soybeans, which is applied in the direction of carboxylic acid ester preparation, fatty acid esterification, chemical instruments and methods, etc., can solve the problem of low activity of epoxide groups in epoxidized soybean oil, which is difficult to remove Eliminate problems such as acrylic acid and corrosion, and achieve the effects of avoiding the use of petrochemical products, easy implementation and control, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
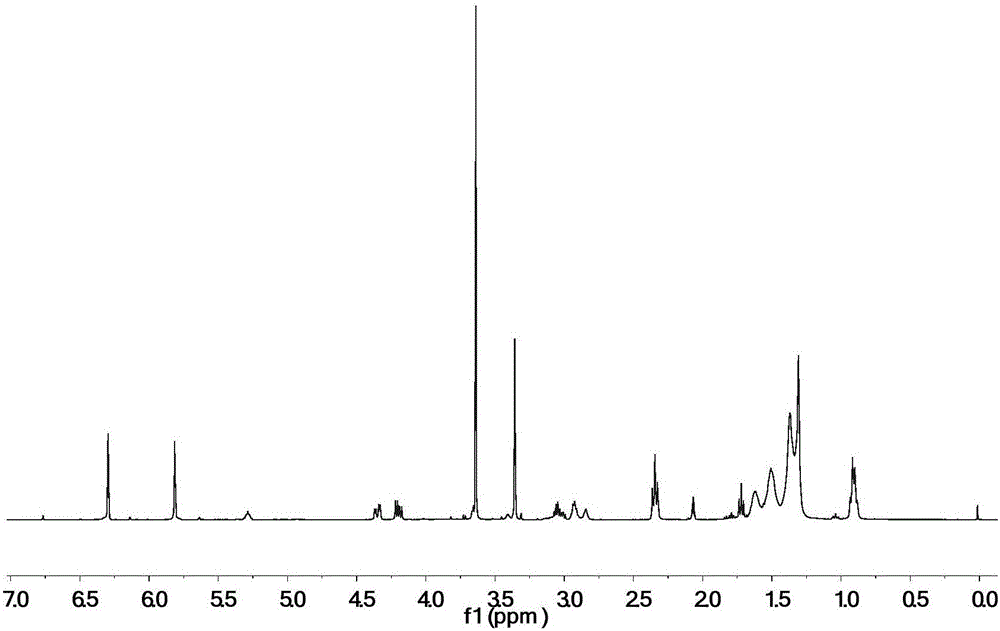
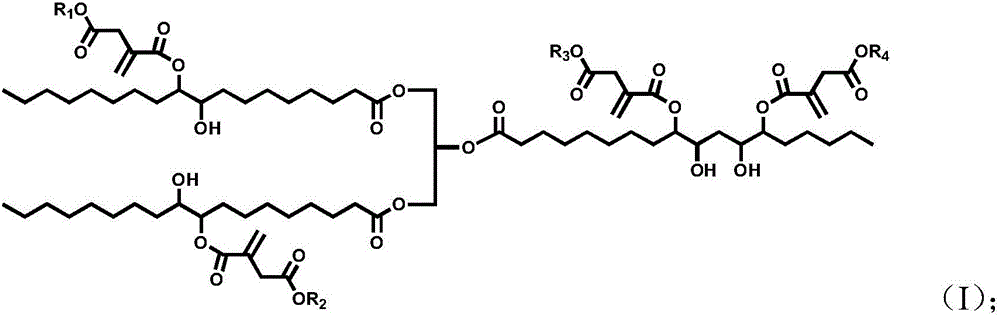
[0032] 100 parts by mass of epoxidized soybean oil, 55 parts by mass of monoethyl itaconate, 4 parts by mass of tetrabutylammonium bromide and 0.5 parts by mass of hydroquinone were uniformly mixed, heated and stirred, and reacted at 110° C. for 2 hours. Treat that reaction is finished, with dichloromethane the crude reaction product is dissolved and washed with water, after decompression distillation removes solvent and moisture, obtains final product, the product 1 The peak at 5.8-6.3ppm in the HNMR spectrogram corresponds to the H on the double bond in the itaconic acid structure, and other peaks are consistent with the H proton displacement in the epoxy soybean oil itaconate structure, proving that the obtained product is Epoxy soybean oil itaconate, the structural formula is as follows:
[0033]
Embodiment 2
[0035] Mix 100 parts by mass of epoxidized soybean oil, 70 parts by mass of monomethyl itaconate, 5 parts by mass of cetyltrimethylammonium chloride and 0.5 parts by mass of 2,5-di-tert-butylhydroquinone Afterwards, heat and stir, and react at 150° C. for 0.5 h. Treat that reaction is finished, with dichloromethane with reaction crude product is dissolved and washed with water, after decompression distillation removes solvent and moisture, obtain final product, the product 1 The peak at 5.8-6.3ppm in the HNMR spectrogram corresponds to the H on the double bond in the itaconic acid structure, and other peaks are consistent with the H proton displacement in the epoxy soybean oil itaconate structure, proving that the obtained product is Epoxy soybean oil itaconate, the structural formula is as follows:
[0036]
Embodiment 3
[0038] 100 parts by mass of epoxidized soybean oil, 10 parts by mass of itaconic acid, 40 parts by mass of monobutyl itaconate, 8 parts by mass of triphenylphosphine and 0.1 parts by mass of p-hydroxyanisole, 0.1 parts by mass of 2-tert-butyl After mixing the base hydroquinone evenly, heat and stir, and react at 30°C for 5h. After the reaction is finished, the crude reaction product is dissolved with dichloromethane and washed with water, and the solvent and moisture are removed by distillation under reduced pressure to obtain the final product. The peak at 5.8-6.3 ppm in the 1HNMR spectrogram of this product corresponds to the structure of itaconic acid The H on the double bond, plus other peaks are consistent with the H proton displacement in the epoxy soybean oil itaconate structure, which proves that the obtained product is epoxy soybean oil itaconate, and the structural formula is as follows:
[0039]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com