Preparation method of Raltitrexed
A technology of raltitrexed and methanol, applied in the field of preparation of raltitrexed, can solve problems such as excessive ignition residue, and achieve the effects of improving quality, high product yield and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 (reaction solvent is methanol and water)
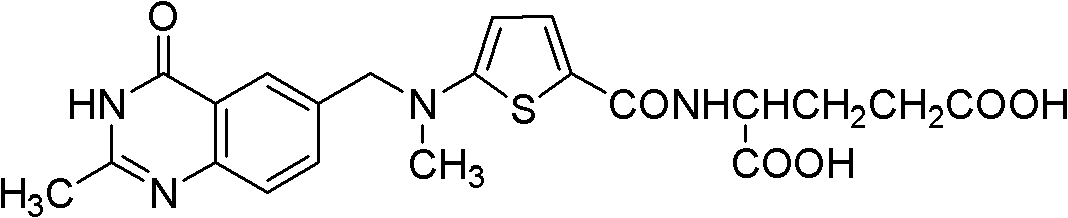
[0026] 40g (0.07mol) N-[[5-[(1,4-dihydro-2-methyl-4-oxo-6-quinazoline)methyl]formyl-]-2-thiophene]formyl ]-L-diethyl glutamate, 1000ml 1M NaOH solution, 142ml methanol was added to the reaction flask, and the internal temperature was maintained at about 0°C, stirred for 2 hours, filtered, and the filtrate was extracted with dichloromethane (2×240ml), and water Layer with 4N hydrochloric acid to adjust the pH, when the pH is adjusted to 6, a small amount of viscous solids are precipitated, stop adding hydrochloric acid, keep stirring for 2 hours, continue to drop hydrochloric acid to adjust the pH to 2.5 after the viscous solid is completely solidified, Stir at this temperature for 10 hours. Filter and wash the solid with purified water until the filtrate is free of Cl - (Detected by silver nitrate), the crude product was obtained as a loose light yellow-green powder solid.
[0027] Put the crude product in a 100...
Embodiment 2
[0028] Embodiment 2 (reaction solvent is ethanol and water)
[0029] 40g (0.07mol) N-[[5-[(1,4-dihydro-2-methyl-4-oxo-6-quinazoline)methyl]formyl-]-2-thiophene]formyl ]-L-diethyl glutamate, 1000ml 1M NaOH solution, 142ml ethanol were added to the reaction flask, and the internal temperature was maintained at about 0°C, stirred for 2 hours, filtered, and the filtrate was extracted with dichloromethane (2×240ml), and water Layer with 4N hydrochloric acid to adjust the pH, when the pH is adjusted to 6, a small amount of viscous solids are precipitated, stop adding hydrochloric acid, keep stirring for 2 hours, continue to drop hydrochloric acid to adjust the pH to 2.5 after the viscous solid is completely solidified, Stir at this temperature for 10 hours. Filter and wash the solid with purified water until the filtrate is free of Cl - (Detected by silver nitrate), the crude product was obtained as a loose light yellow-green powder solid.
[0030] The refining process of the cru...
Embodiment 3
[0031] Embodiment 3 (reaction solvent is water, does not contain organic solvent)
[0032] 40g (0.07mol) N-[[5-[(1,4-dihydro-2-methyl-4-oxo-6-quinazoline)methyl]formyl-]-2-thiophene]formyl ]-L-diethyl glutamate, 1000ml 1M NaOH solution, keep the inner temperature at about 0°C, stir for 2 hours, filter, extract the filtrate with dichloromethane (2×240ml), take the aqueous layer and adjust the pH with 4N hydrochloric acid, When the pH is adjusted to 6, a small amount of viscous solid precipitates, stop adding hydrochloric acid, keep stirring for 2 hours, the viscous solid does not solidify, continue to add hydrochloric acid until the pH is 2.5, the precipitated solid condenses into a strong viscous large Lumpy solids adhered to the stirring paddles, making it impossible to stir. After filtering, the product was washed with distilled water to obtain a viscous crude product.
[0033] The refining process of the crude product is the same as in Example 1, the residue on ignition is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com