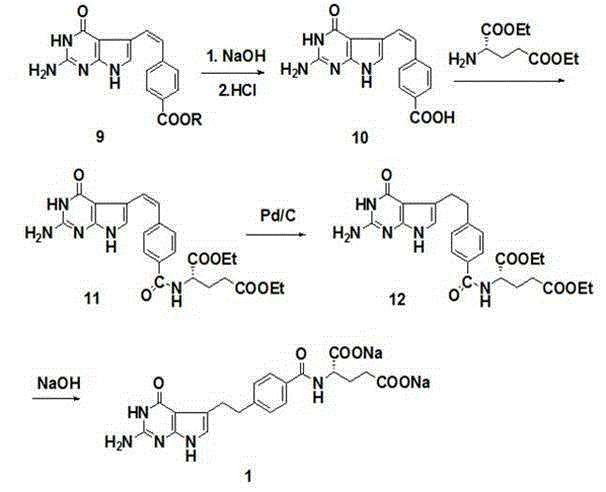
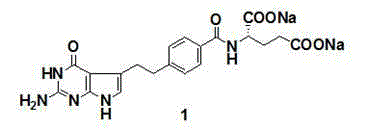
Preparation method of pemetrexed disodium
A kind of technology of pemetrexed disodium and dialkoxy, applied in the field of organic medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
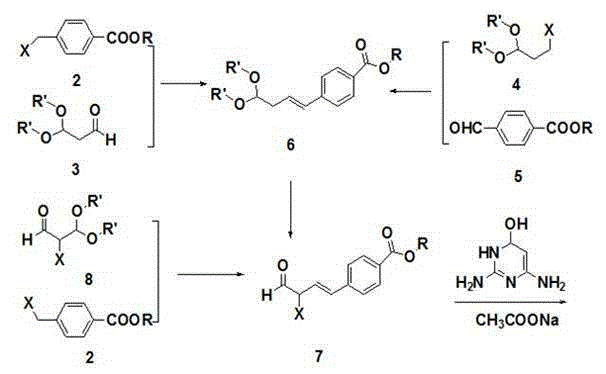
[0037] Methyl bromomethylbenzoate (228.0g, 1.00mol), triphenylphosphine (288.2g, 1.10mol) and tetrahydrofuran (1.2L) were placed in a three-necked flask and stirred at 25°C for 2h. The reaction solution was cooled to 5°C, potassium tert-butoxide (112 g, 1.00 mol) was added in batches, and stirring was continued at room temperature for 2 h after the addition was complete. Recool the reaction solution to 5°C, add dropwise a mixture of 3,3-dimethoxypropanal (118.0 g, 1.00 mol) and tetrahydrofuran (118 mL), and continue stirring at room temperature for 5 h after the dropwise addition. Recover solvent THF, add ethyl acetate, wash with hot water, dry, recover ethyl acetate to obtain methyl 4-(4,4-dimethoxy-1-butenyl)benzoate (237.5g, 95%) .
Embodiment 2
[0039] Put 1,1-dimethoxy-3-bromopropane (182.0g, 1.00mol), triphenylphosphine (288.2g, 1.10mol) and tetrahydrofuran (0.9L) in a three-necked flask and stir at 25°C 2h. The reaction solution was cooled to 5°C, potassium tert-butoxide (112 g, 1.00 mol) was added in batches, and stirring was continued at room temperature for 2 h after the addition was complete. Recool the reaction solution to 0-5°C, add a mixture of methyl p-aldehyde benzoate (164.0 g, 1.00 mol) and tetrahydrofuran (164 mL) dropwise, and continue stirring at room temperature for 5 h after the dropwise addition. Recover solvent THF, add ethyl acetate, wash with hot water, dry, recover ethyl acetate to obtain methyl 4-(4,4-dimethoxy-1-butenyl)benzoate (240.0g, 96%) .
Embodiment 3
[0041] Place 4-(4,4-dimethoxy-1-butenyl)methyl benzoate (200g, 0.80mol) and 1,2-dichloroethane (1L) in a three-necked flask and cool to 5 ℃, N-bromosuccinimide (155.7 g, 0.88 mol) was added in batches, and stirring was continued at room temperature for 5 h after the addition was complete. Re-cool the reaction solution to 5°C, add dilute hydrochloric acid (1mol / L) dropwise, stir for 0.5h, separate the layers, wash the organic layer with saturated sodium bicarbonate, wash with water, wash with saturated brine, dry, and recover the solvent to obtain 4- Methyl (3-formyl-3-bromo-1-propenyl)benzoate (146.6g, 65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com