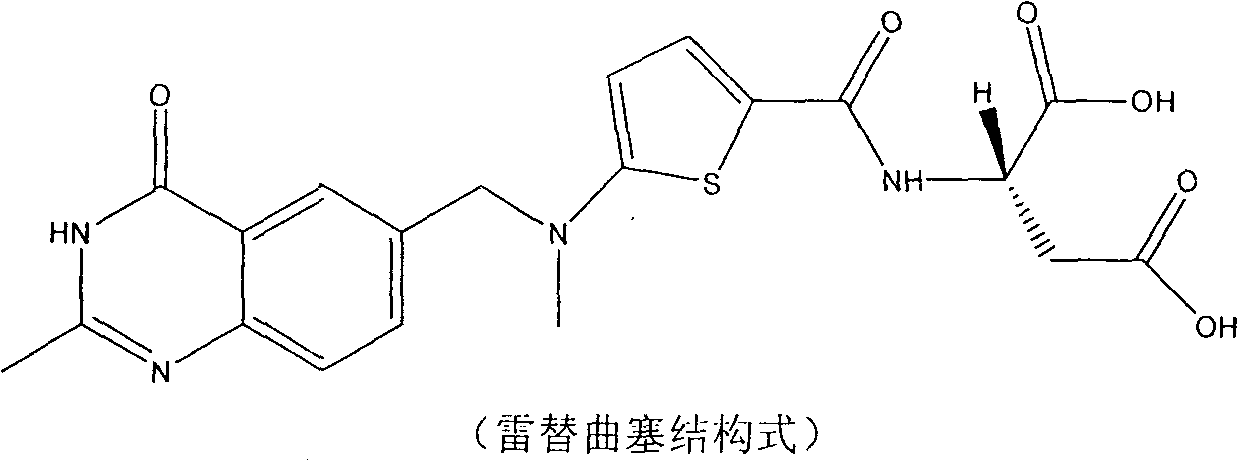
Method for industrial preparation of raltitrexed and novel raltitrexed crystal form for pharmacy
A technology of raltitrexed and process scheme, which is applied in the field of synthesis and preparation of the antineoplastic drug raltitrexed, and can solve the problems of consuming large elution solvents and difficulty in preparing a large amount of raltitrexed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1), N-[5-[N-(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)-methyl]-N-methyl]-2- Synthesis of Diethyl Thiophenoyl-L-Glutamate (LT12)
[0024] I. Reaction formula:
[0025]
[0026] II. Feeding table: (Table 2)
[0027]
[0028] III. Operation:
[0029]Suspend 5.1g (20.2mmol) LTS2 in 40ml of DMF dried with 4A molecular sieves, add 12g (142.9mmol) of sodium bicarbonate into a stirred 500ml three-necked flask equipped with a thermometer, stir for 15 minutes, and then Add 7.6g (22.2mmol) of LTS1 dissolved in 40ml DMF in advance into the reaction flask, heat up to 40°C under nitrogen protection, react for 24 hours, add 6g of sodium bicarbonate, and continue the reaction for about 12 hours under nitrogen protection, (with The basic complete reaction of the bromide on the spot plate is the basis for judging. TLC detects the developing agent condition: ethyl acetate / methanol=10 / l), stops the reaction, cools to room temperature, adds 240 ml of dichloromethane, stirs for ha...
Embodiment 2
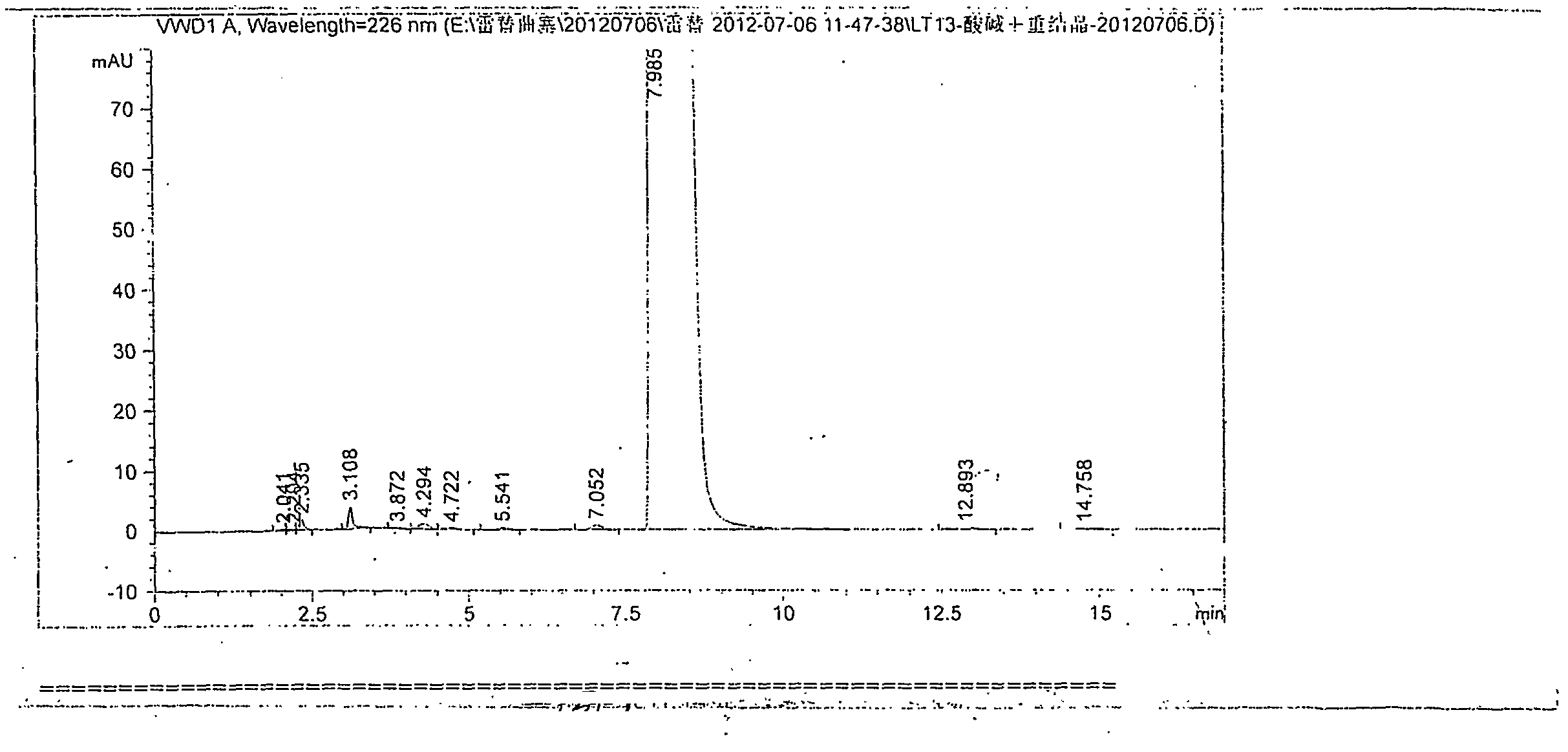
[0046] The catalyst used in the first step reaction was changed to 2,6-lutidine, and the equivalent used was 5 times that of LTS2. The rest of the operations were basically the same as in Example 1, and then LT12 with a purity of 95.7% and a yield of 61% was obtained. Using LT12 of this purity, raltitrexed was obtained through alkali hydrolysis and acid analysis, the operation was the same as in Example 1, and finally the finished product of raltitrexed was obtained, the total yield of the second step reaction and the third step refining was 76%, the product The purity reaches 99.81%, without impurities greater than 0.1%.
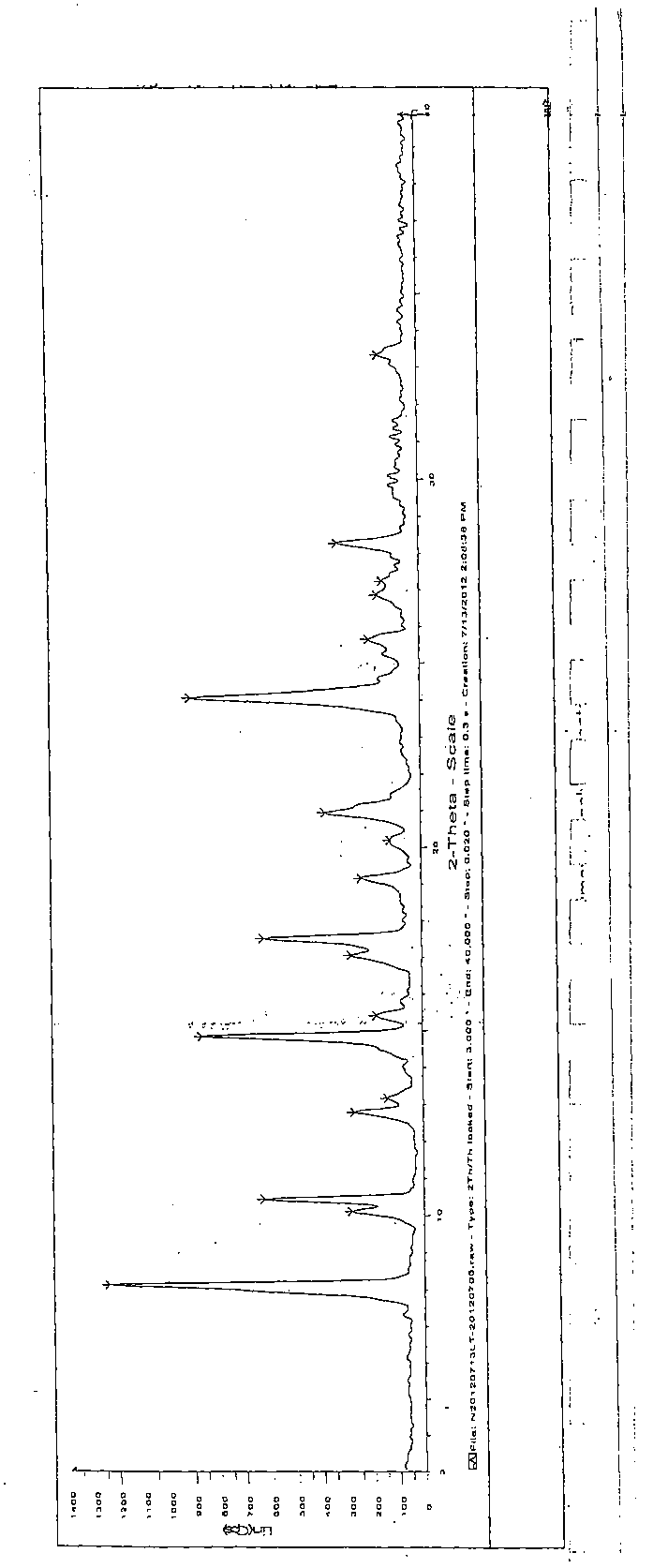
[0047] (4) Determination of the crystal form of raltitrexed
[0048] Through X-ray diffraction analysis, it can be seen from the spectrum that the obtained raltitrexed is a crystalline compound, and the characteristic diffraction 2θ angle values are: 8.066, 10.059, 10.389, 12.751, 13.129, 14.810, 15.390, 17.032, 17.486, 19.141, 20.930, 24.047 , 25.647, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com