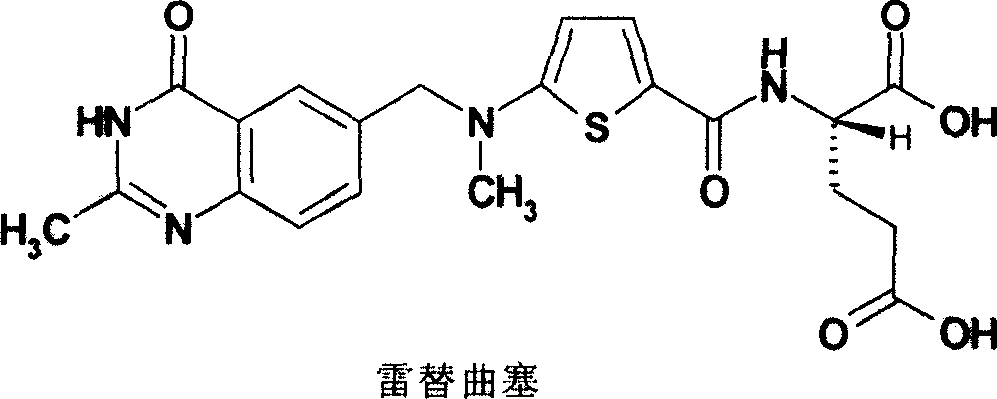
Improved process of preparing Raltitrexed
A technology of raltitrexed and a process method, which is applied in the field of medicine, can solve the problems of restricting industrialization, high toxicity of bromine, and low process yield, and achieve the effects of reducing solvent consumption and solving toxicity and safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Preparation of N-(5-methylaminothiophene-2-formyl)-L-glutamic acid diethyl ester
[0014] (N-(5-aminothiophene-2-formyl)-L-glutamic acid diethyl ester) (60g,), 2,6-lutidine (29.35g, 19.5ml,), DMF ( 150ml), the temperature was raised and protected by nitrogen gas. Iodomethane (25.98g, 11.43ml) was added dropwise at a temperature of about 50°C and protected from light. After the dropwise addition was completed, the reaction was maintained at 100° C. for 24 hours. After the heat preservation is completed, lower the temperature, add 500ml of water, and extract with ethyl acetate (3×500ml); combine the organic layers, wash with saturated brine (2×500ml), dry over anhydrous magnesium sulfate, and evaporate the organic solvent under reduced pressure to obtain a brown color 57.1 g of oily product, yield: 91.2%.
Embodiment 2
[0015] Example 2: Preparation of (N-(5-aminothiophene-2-formyl)-L-diethyl glutamate)
[0016] Put (N-(5-nitrothiophene-2-formyl)-L-glutamic acid diethyl ester) (180g), methanol (500ml) and water (500ml) in sequence in a 2000ml three-necked bottle, sodium sulfide (195g) was slowly heated up to 70°C, and kept for 5 hours for reaction. TLC (developing agent: ethyl acetate: n-hexane = 2: 1) detects the end point of the reaction. After the reaction solution has no raw material spots, stop the reaction, add diatomaceous earth, filter with suction, concentrate the filtrate to dryness, and then add 500 ml of dichloromethane. Wash twice with water, dry over anhydrous magnesium sulfate, filter with suction, and concentrate the filtrate under reduced pressure to obtain 135 g of brown oil, yield: 82.1%.
Embodiment 3
[0017] Example 3: N-[5-[N-methyl-N-(2-methyl-4-oxo-3,4-dihydroquinoline-6-methyl)amino]-2-thiophenoyl Preparation of ]-L-diethyl glutamate
[0018] Diethyl N-(5-methylaminothiophene-2-formyl)-L-glutamate (25g), 2,6-lutidine (7.82g,), DMF (430ml) and 2- Methyl-6-bromomethyl-4-oxo-3,4-dihydroquinazoline (21.44g) was added into a 1000ml reaction flask, the temperature was raised and protected by nitrogen, and the reaction was kept at 80°C-85°C for 18 hours. After the heat preservation was completed, DMF was evaporated under reduced pressure. Cool down, add 500ml of water, and extract with ethyl acetate (3×600ml); combine the organic layers, wash with saturated brine (2×500ml), dry over anhydrous magnesium sulfate, and distill off the organic solvent under reduced pressure, the residue in Add 60ml of methanol, move it to a reaction bottle, slowly add 300ml of ether dropwise under stirring, crystallize, and filter with suction to obtain 35.8g of the product, yield: 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com