Method for preparing raltitrexed intermediate, i.e., N-(5-methylamino-2-thiophene formyl)-L-glutamate diethyl ester
A technology of diethyl glutamate and thiophenoyl, which is applied in the field of medicine, can solve the problems of many side reactions, difficult to control the reaction, and low yield, achieve mild reaction conditions, solve the problems of toxicity and safety, and operate simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
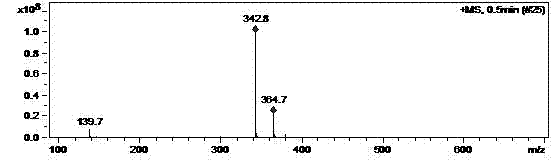
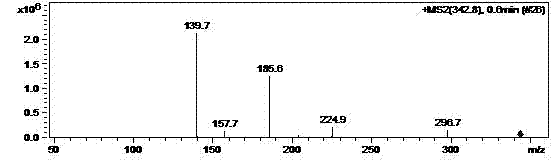
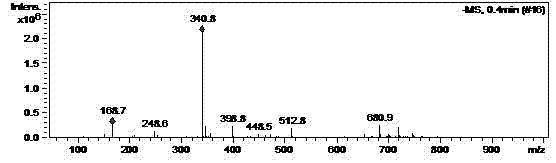
[0027] Dissolve 4.2g of N-(5-amino-2-thiophenoyl)-L-glutamic acid diethyl ester in 100ml of ethyl acetate, add 4.0g of paraformaldehyde, stir at 50°C for 24 hours, filter, reduce The concentrated oil was dissolved in 50ml of absolute ethanol and cooled to 0°C, 1.0g of sodium borohydride was added in batches, stirred at room temperature for 5 hours, water was added to terminate the reaction, concentrated to dryness under reduced pressure, and 100ml of water and 100ml of dihydrogen were added. Chloromethane was separated into layers, and the organic phase was dried over anhydrous magnesium sulfate to obtain 3.0 g of N-(5-methylamino-2-thienoyl)-L-glutamic acid diethyl ester with a yield of 68.4%. The above products are supplied by Figure 1~4 Its authenticity can be verified.
Embodiment 2
[0029] Dissolve 4.2g of N-(5-amino-2-thiophenoyl)-L-glutamic acid diethyl ester in 100ml of ethyl acetate, add 10.0g of formaldehyde solution, stir at 50°C for 24 hours, filter, and depressurize Concentrated oil, dissolved in 50ml of absolute ethanol and cooled to 0°C, added 1.0g of sodium borohydride in batches, stirred at room temperature for 5 hours, added water to terminate the reaction, concentrated to dryness under reduced pressure, added 100ml of water and 100ml of dichloro Methane was separated, and the organic phase was dried over anhydrous magnesium sulfate to obtain 3.5 g of N-(5-methylamino-2-thienyl)-L-glutamic acid diethyl ester with a yield of 79.9%.
Embodiment 3
[0031] Dissolve 4.2g of N-(5-amino-2-thiophenoyl)-L-glutamic acid diethyl ester in 100ml of ethyl acetate, add 4.0g of paraformaldehyde, stir at 50°C for 24 hours, filter, reduce The concentrated oil was dissolved in 50ml of absolute ethanol and cooled to 0°C, 1.0g of potassium borohydride was added in batches, stirred at room temperature for 5 hours, water was added to terminate the reaction, concentrated to dryness under reduced pressure, and 100ml of water and 100ml of dihydrogen were added. Chloromethane was separated into layers, and the organic phase was dried over anhydrous magnesium sulfate to obtain 2.8 g of N-(5-methylamino-2-thienoyl)-L-glutamic acid diethyl ester with a yield of 63.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com