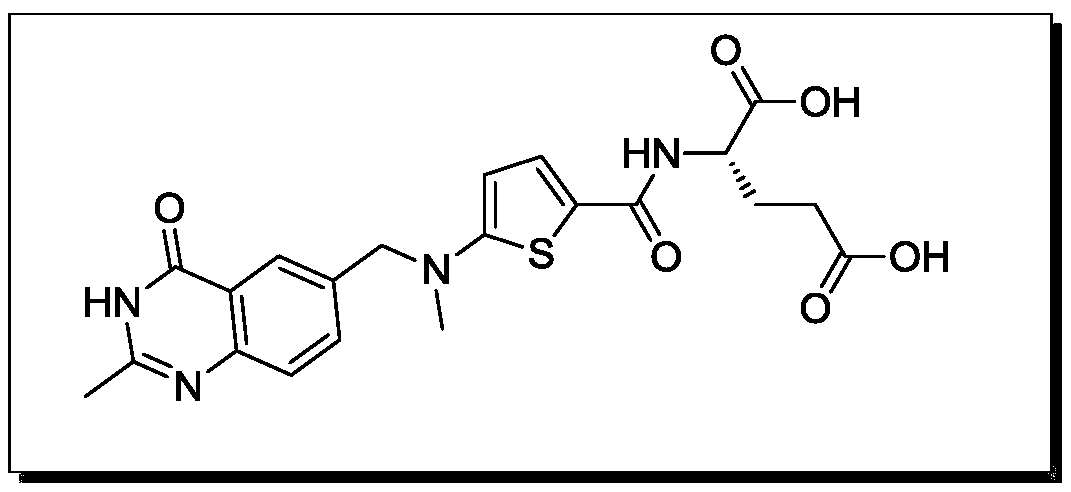
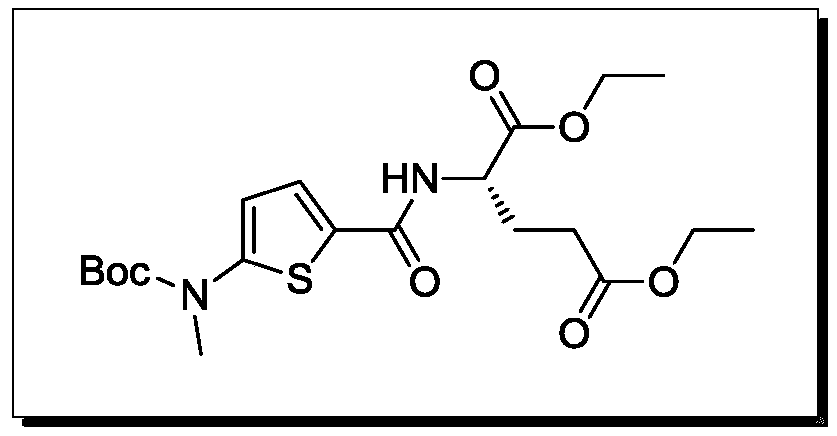
A kind of synthesis technique of raltitrexed key intermediate
A technology of raltitrexed and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of complex process route, high purification difficulty, unfavorable industrialization, etc., and achieve the effects of improving process yield, shortening process cycle and reducing preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
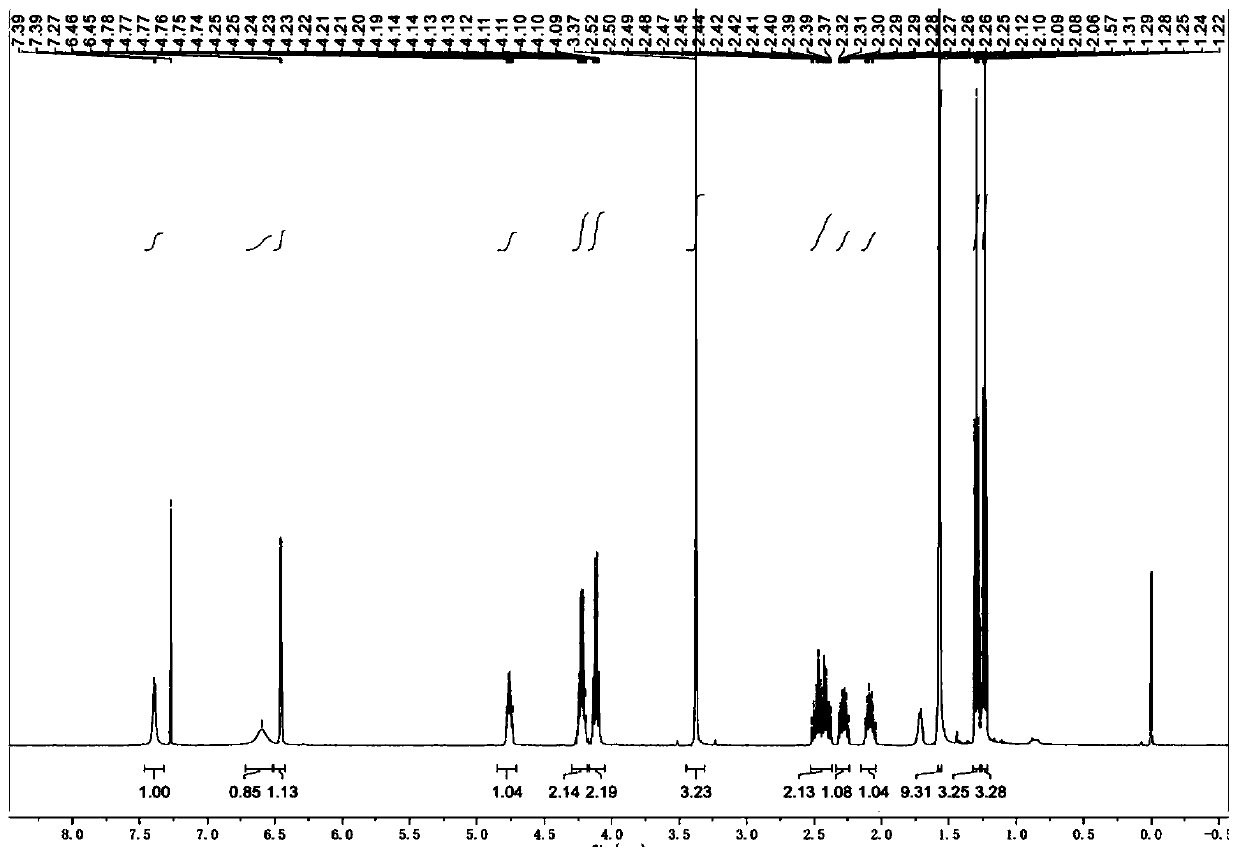
[0040] Step 1: Preparation of Diethyl Glutamate in Isocyanate Solution
[0041] Add diethyl glutamate hydrochloride (100.0 g, 0.42 mol) and 400 mL of dichloromethane into a 1 L reaction flask, replace with nitrogen three times, cool down (internal temperature drops to -5°C to 5°C), and set aside.
[0042] In a 250ml reaction flask, under nitrogen protection, dichloromethane (100mL) and triphosgene (41.5 g, 0.14mol) were added in sequence, and stirred to dissolve. The dichloromethane solution of triphosgene was slowly dropped into the above-mentioned diethyl glutamate hydrochloride dichloromethane suspension, the internal temperature was controlled at -5°C to 5°C, and the system was stirred for 30 minutes after the addition was completed. Another triethylamine (92.8g, 0.92mol) was taken, and the internal temperature was controlled at -5°C to 5°C, slowly dropped into the reaction solution, and after the addition was completed, the reaction was stirred at 0°C to 10°C for 2 hours,...
Embodiment 2
[0048] Step 1: Preparation of Diethyl Glutamate in Isocyanate Solution
[0049] Add diethyl glutamate hydrochloride (100.0 g, 0.42 mol) and 400 mL of dichloromethane into a 1 L reaction flask, replace with nitrogen three times, cool down (internal temperature drops to -5°C to 5°C), and set aside.
[0050] 250mL reaction flask, under the protection of nitrogen, add dichloromethane (100mL) and diphosgene (27.7 g, 0.14mol) in sequence, stir to dissolve. Slowly add the diphosgene dichloromethane solution dropwise into the diethyl glutamate hydrochloride dichloromethane suspension, control the internal temperature -5°C to 5°C, and stir the system for 30 minutes after the addition. Another triethylamine (92.8g, 0.92mol) was taken, and the internal temperature was controlled at -5°C to 5°C, slowly dropped into the reaction solution, and after the addition was completed, the reaction was stirred at 0°C to 10°C for 2 hours, and the reaction was detected by TLC. completely.
[0051] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com