A kind of pharmaceutical composition of pemetrexed disodium
A technology of pemetrexed disodium and composition, applied in the field of freeze-dried medicinal compositions, can solve problems such as lack
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9 and comparative example 1~8
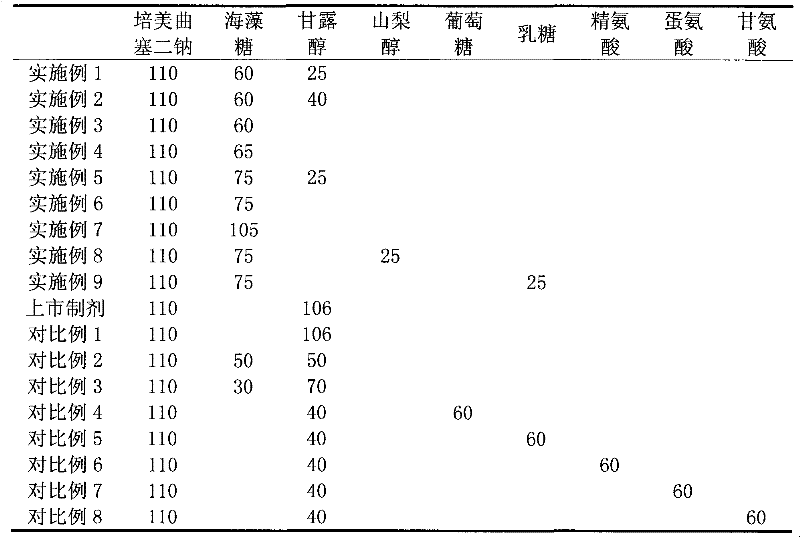
[0036] The freeze-dried products of Examples 1-9 and Comparative Examples 1-8 were prepared according to the following methods.
[0037]Preparation method: Weigh the corresponding pemetrexed disodium raw materials and auxiliary materials as shown in Table 1, add sterilized water for injection to 2.5L, stir and dissolve, adjust the pH value of the solution with 0.1N sodium hydroxide solution or hydrochloric acid solution To 7.5, add 2.29 g of activated carbon for needles, stir, filter, and then filter the filtrate with a 0.22 μm microporous membrane, and distribute it into type I glass bottles. Freeze in a freeze-drying cabinet to about -40°C for 4 hours, sublimate and dry at -30 to -20°C for 30 hours, and then dry at 25°C for 12 hours, fill with nitrogen, plug, take out of the box, seal with a gland, and pack to get the example 1-9 and the pemetrexed disodium lyophilized powder for injection of comparative examples 1-8.
[0038] Table 1 Embodiment 1~9, comparative examples 1~...
Embodiment 10
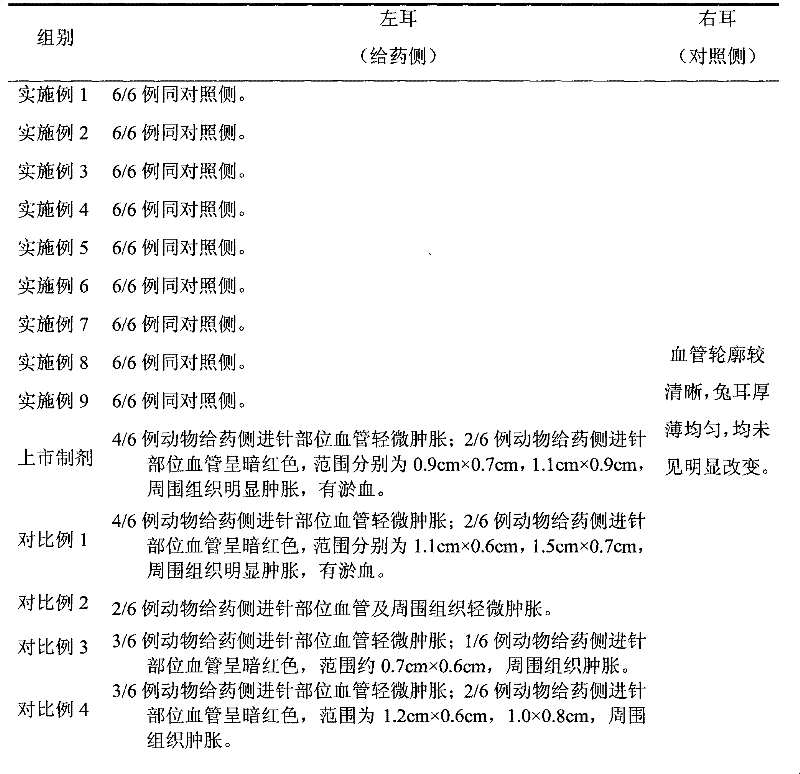
[0041] Example 10: Vascular stimulation test of powder injections prepared according to Examples 1-9 and Comparative Examples 1-8 and pemetrexed disodium freeze-dried powder injections on the market
[0042] 1. Test method: Take 108 New Zealand rabbits qualified for quarantine, weighing 2.5-3kg, half male and half male, and divide them into 18 groups on average, with 6 rabbits in each group, and prepare according to the methods of Examples 1-9 and Comparative Examples 1-8 respectively Pemetrexed disodium powder injection and listed pemetrexed disodium powder injection, using the same body left and right sides of the self-contrast method, the rabbit's left ear vein was given the corresponding injection of pemetrexed disodium solution, the concentration is 1mg / ml, approximately equivalent to clinical It is planned to use 1.2 times the concentration of the maximum intravenous administration, give normal saline to the vein of the right ear margin of the rabbit, the administration v...
Embodiment 11
[0067] Example 11: Muscle stimulation test of powder injections prepared according to Examples 1-9 and Comparative Examples 1-8 and marketed preparations
[0068] 1. Test method: 108 New Zealand white rabbits were shared with the vascular stimulation test, grouped into the same blood vessel stimulation test, using the same body left and right muscle self-comparison method, the left quadriceps muscle was given the test substance, and the administration concentration was 1mg / mL ( It is approximately equivalent to 1.2 times the concentration of intravenous administration intended for clinical use), the administration volume is 1.0mL / side, and an equal volume of normal saline is administered to the right side as a control, administered once every 2 days, a total of 2 times. Necropsy was performed 48 hours and 14 days after the last administration, respectively.
[0069] 2. Observation indicators
[0070] Weighing of animals: Animals were divided into groups at the end of the quar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com