Method for preparing improved intermediate for producing high-purity pemetrexed and method for producing high-purity pemetrexed using intermediate
A technology of pemetrexed diacid and pemetrexed diethyl ester, which is applied to pemetrexed diethyl ester or prepared high-purity, ground, used in the field of preparation, can solve the problem of low-purity by-products of products and cannot improve the level And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
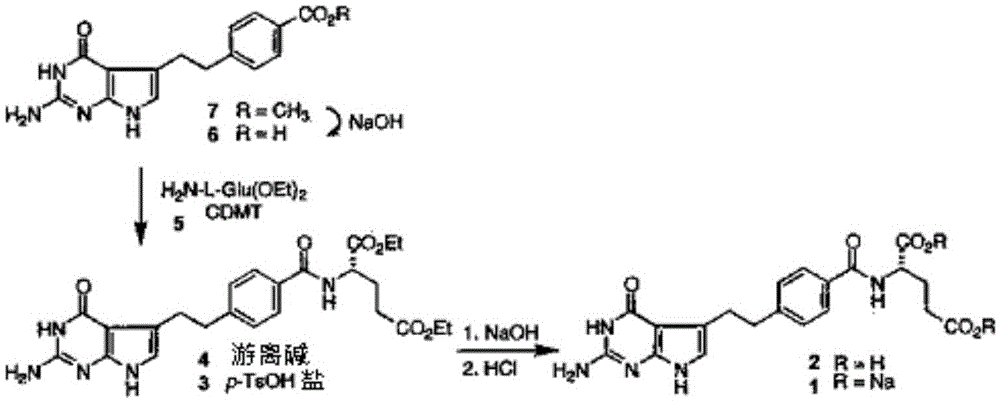
[0095] Embodiment 1: the preparation of pemetrexed diethyl ester p-toluenesulfonate (chemical formula 3)
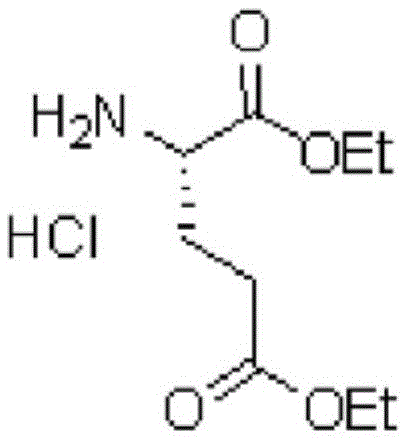
[0096] Add 1 L of saturated NaHCO to the reactor 3 Aqueous solution and 88 g of L-glutamic acid diethyl ester hydrochloride (LGA), and after stirring at room temperature for 30 minutes, 1 L of DCM was thrown in, and the organic layer was extracted. After adding 0.5 L of DMF to the separated organic layer, it was concentrated at 20 to 30° C. to remove more than 80% of DCM. Put 93g of 4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzene into the concentrate Formic acid (chemical formula 6, HPLC purity is 98.3%), and after cooling to 0 ~ 10 ° C, 93g of N-methylmorpholine (NMM) and 65g of 2-chloro-4,6-dimethoxy- 1,3,5-Triazine. After stirring the reaction solution at 5-15° C. for 1 hour, 1 L of DCM and 1 L of purified water were added, and the organic layer was extracted (HPLC purity: 97.9%). 0.3 L of DMF, 3.3 L of EtOH, and 148 g of p-toluenesulf...
Embodiment 2
[0099] Embodiment 2: the preparation of pemetrexed diacid (chemical formula 2)
[0100] 1 L of 1N NaOH aqueous solution was added to the reactor, and 143 g of the compound represented by Chemical Formula 3 prepared in Example 1 was added at 5-15°C. After stirring at 5-15° C. for 2 hours, it was filtered (HPLC purity: 99.8%). 2 L of EtOH was added to the filtrate, and a 2N HCl aqueous solution was slowly added dropwise at 5 to 15° C. to adjust the pH to 3.0. After stirring the resulting crystalline mixture at 40-50°C for 1 hour, it was filtered at 40°C. The filtrate was washed with 2 L of purified water, and further washed with 1 L of EtOH. The obtained filtrate was put into 4 L of EtOH / purified water (1:1, v / v), and the mixture was stirred at 40-50° C. for 1 hour, cooled, and filtered at normal temperature. The filtrate was washed with 2 L of purified water, and further washed with 1 L of EtOH. Vacuum drying was performed at 40-50° C. for 16 hours to obtain 88 g of pemetre...
Embodiment 3
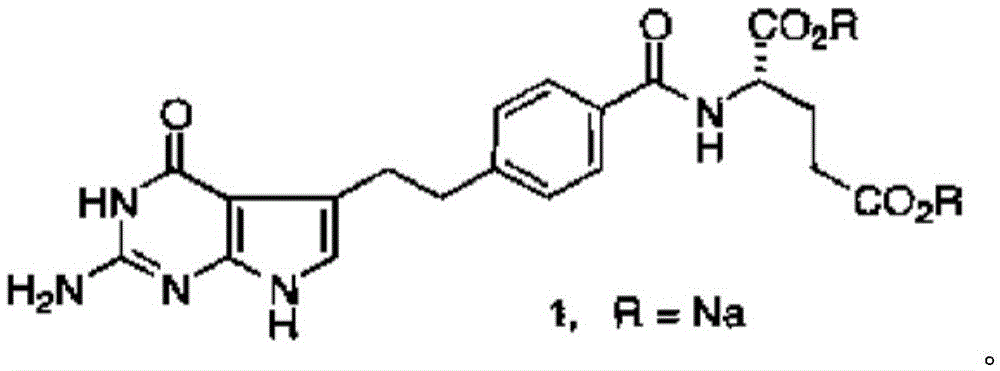
[0101] Embodiment 3: the preparation of pemetrexed disodium salt (chemical formula 1)
[0102] 1 L of 1N NaOH aqueous solution was added to the reactor, and 87 g of the compound represented by Chemical Formula 2 prepared in Example 2 was added at 5-15°C. After stirring for 30 minutes, it was filtered, and a 0.5N HCl aqueous solution was slowly added dropwise to the filtrate to adjust the pH to 7.5-8.5. The solution was heated to 50-60° C., and 7 L of EtOH was slowly added dropwise to form crystals. The resulting white solid was cooled slowly to room temperature and filtered. Wash with 1L of EtOH / purified water mixture (4:1, v / v), and carry out vacuum drying at 40° C. for 21 hours, thereby obtaining 91 g of white solid pemetrexed disodium salt (yield: 87%, HPLC purity: >99.9%, various impurities: <0.05%). The water content measured by the Karl Fischer method was 9.05% by weight, so it was confirmed that the disodium salt of pemetrexed was 2.5 hydrate (required standard: 8.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com