Preparation method of pemetrexed disodium
A technology of trifluoropyrimidine and trifluoromethyl, which is applied in the field of preparation of trifluoropyrimidine, can solve the problems of long reaction process, low reaction yield, and difficult wastewater treatment, and achieve the goal of increasing yield and shortening the reaction process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
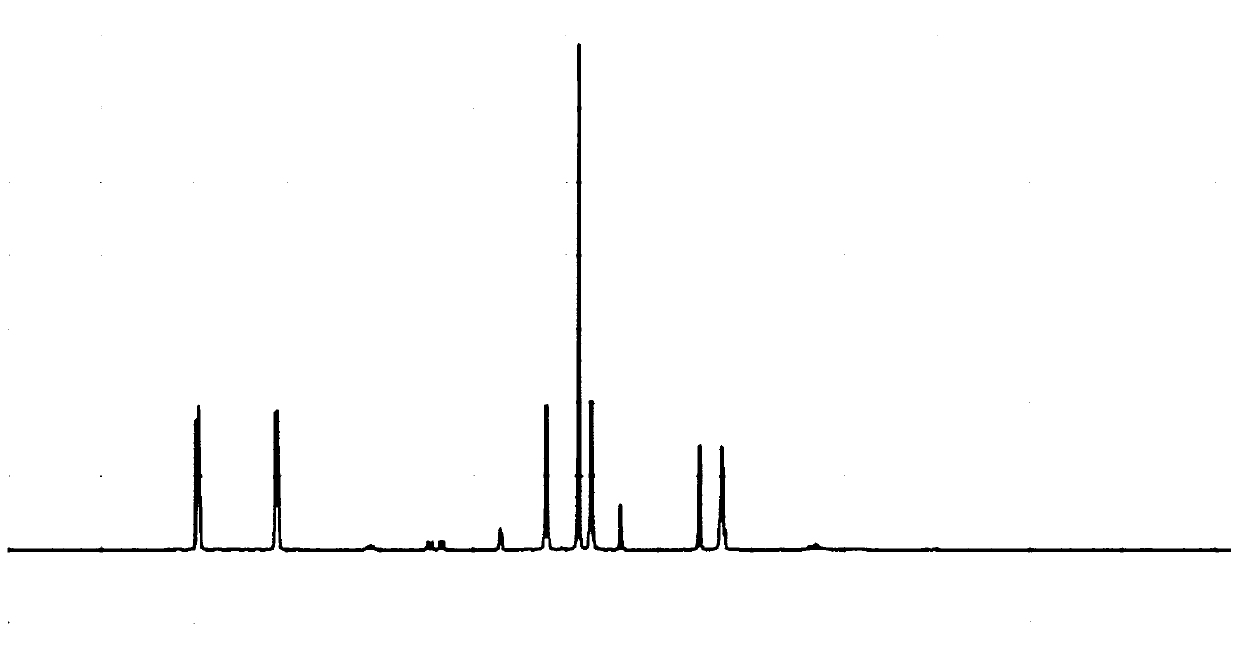
Image
Examples
Embodiment 1
[0030] A preparation method of trifluoropyrim, comprising the following steps:
[0031] (1) Take 2-(3-(trifluoromethyl)phenyl)malonate 30.43g and N-(pyridin-5-ylmethyl)pyridin-2-amine 18.62g, dissolve the two in 0.5 In the N-methylimidazole of L, add 28.06g of tetramethyl chlorourea hexafluorophosphate as catalyst, seal and stir reaction under microwave condition, obtain crude product;
[0032] (2) Remove N-methylimidazole from the crude product under reduced pressure, then add ethyl acetate and stir evenly, and use silica gel column chromatography to obtain the trifluoropyrimidine.
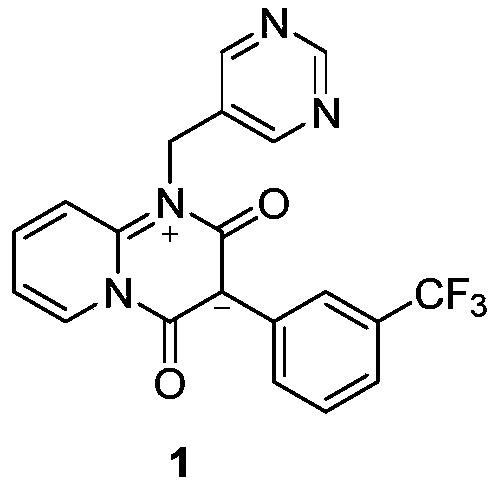
[0033] The synthetic route of the present embodiment is shown in the following formula:
[0034]
[0035] The present invention has the advantage of utilizing 2-(3-(trifluoromethyl)phenyl)malonate and N-(pyridin-5-ylmethyl)pyridin-2-amine to react directly under catalyst and microwave conditions , the reaction process is only one step, which greatly shortens the reaction process and improves...
Embodiment 2
[0041] A preparation method of trifluoropyrim, comprising the following steps:
[0042] (1) Take 2-(3-(trifluoromethyl)phenyl)malonate 30.56g and N-(pyridin-5-ylmethyl)pyridin-2-amine 18.07g, dissolve the two in 0.5 In 1 L of toluene, 28.12 g of tetramethyl chlorourea hexafluorophosphate was added as a catalyst, and the reaction was sealed and stirred under microwave conditions to obtain a crude product;
[0043] (2) Remove the toluene from the crude product under reduced pressure, then add ethyl acetate and stir evenly, and use silica gel column chromatography to obtain the trifluoropyrimidine.
[0044] In this example, the 2-(3-(trifluoromethyl)phenyl)malonate is diethyl 2-(3-(trifluoromethyl)phenyl)malonate, and the solvent is toluene.
[0045] Specifically, the microwave condition is a microwave environment of 4 GHz, the stirring is continued at 300 r / min for 30 min, the column chromatography uses 100 g of silica gel, and the mobile phase used is ethyl acetate chloroform...
Embodiment 3
[0048] This embodiment is basically the same as Embodiment 2, the difference is:
[0049] In the process of removing the solvent under reduced pressure, the environmental vacuum starts from 50mmHg, and increases the environmental vacuum at a rate of 1mmHg / min until the environmental vacuum reaches 100mmHg, and the total duration is 60min .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com