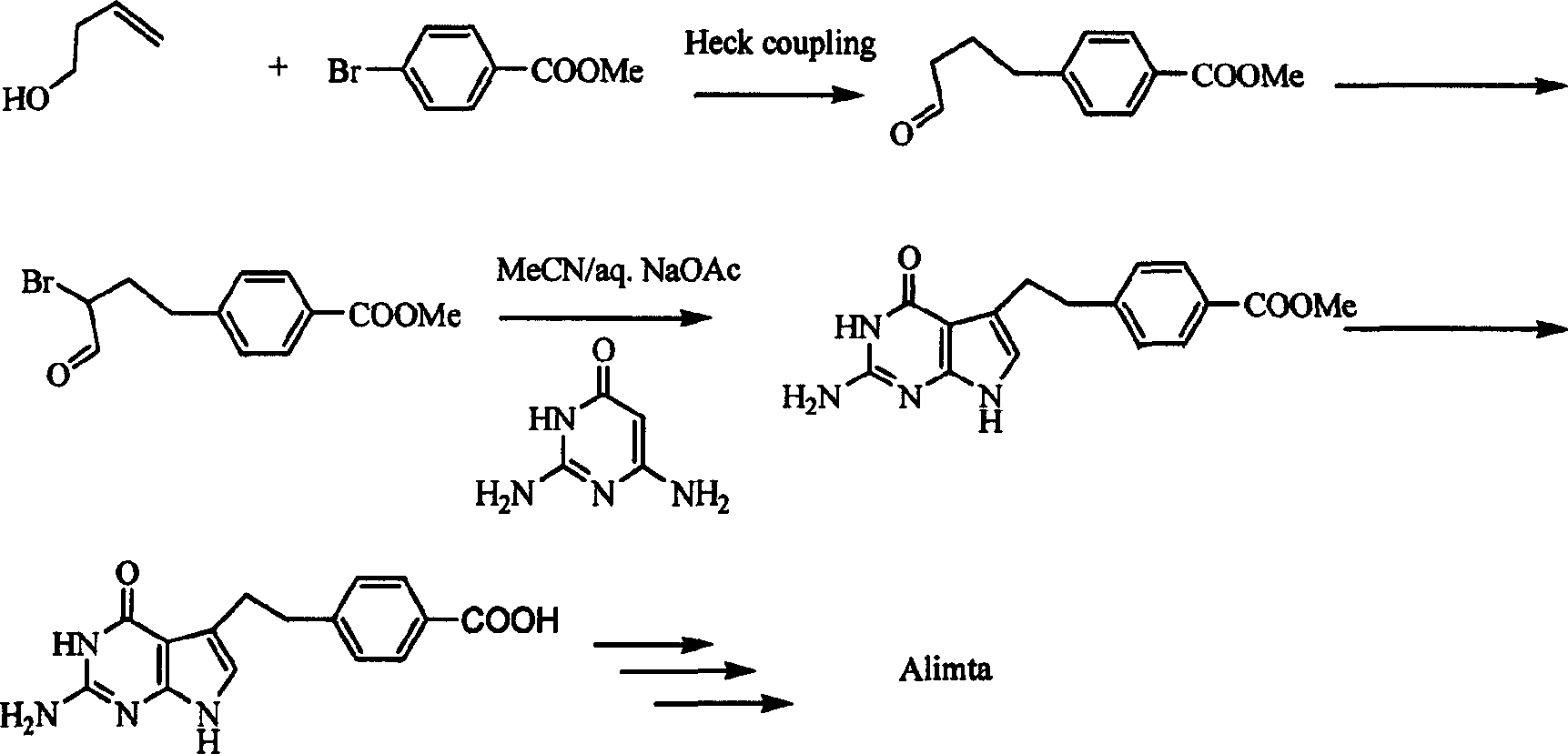
Pemetrexed disodium key intermediate and its synthesis method, and method for synthesizing pemetrexed disodium from the said intermediate
A technology of pemetrexed disodium salt and metrexed disodium salt, which is applied in the field of pemetrexed disodium salt key intermediate and its synthesis, compound and its preparation, and can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
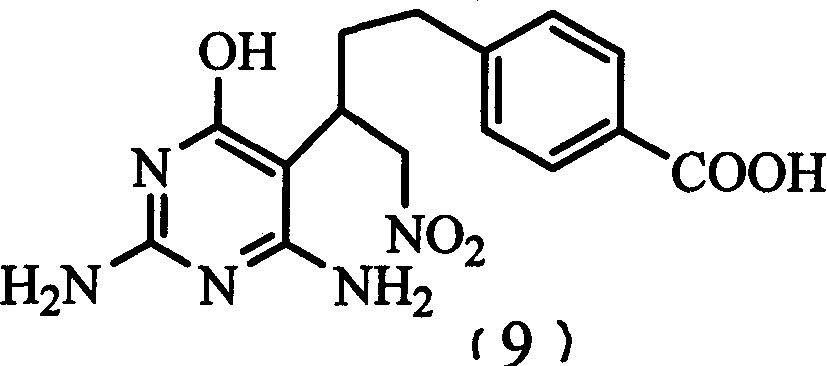
[0026] Embodiment 1: Synthesis of 1-nitro-2-(2,6-diamino-4-(3H) oxopyrimidin-5-yl)-4-(4-carboxyphenyl)-1-butane
[0027] Add 0.1 mole of 1-nitro-4-(4-carboxyphenyl)-1-butene (8) and 0.1 mole of 2,6-diamino-4(3H)oxopyrimidine into the reaction flask, then add NaOH aqueous solution , Insulate the reaction at 50-60°C until the raw materials disappear. Cool, add AcOH to adjust the pH=5-6, precipitate out, and the filter cake obtained by suction filtration is the desired product: 1-nitro-2-(2,6-diamino-4-(3H)oxopyrimidine- 5-yl)-4-(4-carboxyphenyl)-1-butane (9). Yield 80%, Bp=180°C (dec), 1 HNMR: 1.680-2.143 (2H, m, m), 2.501-2.682 (2H, m, m), 3.441 (H, m), 4.753-4.990 (2H, m, m), 6.490 (2H, s), 6.952 (2H, s), 6.948 (2H, d), 7.808 (2H, d).
Embodiment 2
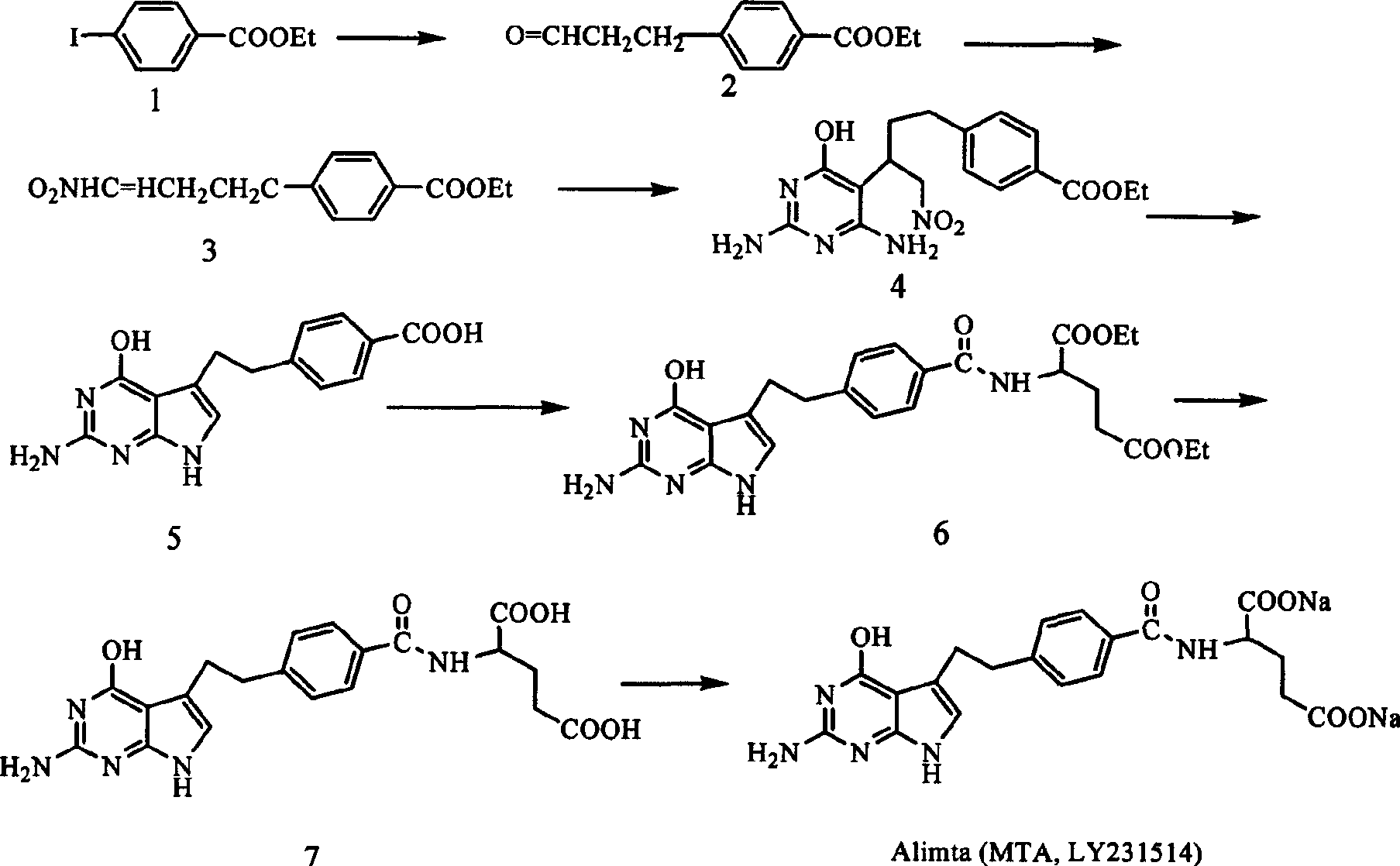
[0028] Example 2: Synthesis of 4-[2-(2-amino-4-(3H)oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid
[0029] Add compound 9 obtained above into the reaction flask, then add sodium hydroxide solution and stir until the solid dissolves. The reaction was incubated at 50°C for 2 hours. Prepare 8N sulfuric acid and cool to below 0°C. Stir and control the reaction temperature below 0°C, add the above reaction solution dropwise into the acid, and then continue the reaction for 3 hours. The pH value of the reaction solution was adjusted to 8 with sodium oxide solution, and the reaction was continued for 1 hour. Glacial acetic acid was added dropwise to generate a precipitate, and the filtered solid was the desired product. 4-[2-(2-Amino-4-(3H)oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid. Yield 75%, 1 H NMR (DMSO-d 6 , 400MHz) δ: 2.83(t, 2H, Bz-CH 2 -), 2.96(t, 2H, Ph-CH 2 -), 6.08(s, 2H, NH 2 ), 6.31 (s, 1H, C=CH-), 7.28, 7.83 (AA'BB', d, d, 4H, C 6 h 4 ), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com