Preparation process for synthesizing high-purity Pemedolac
A preparation process, a technology for pemetrexed acid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
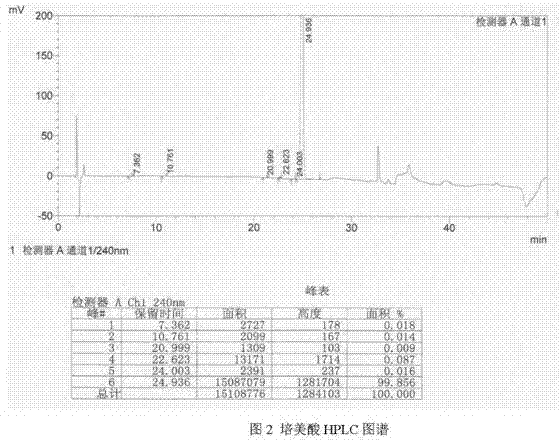
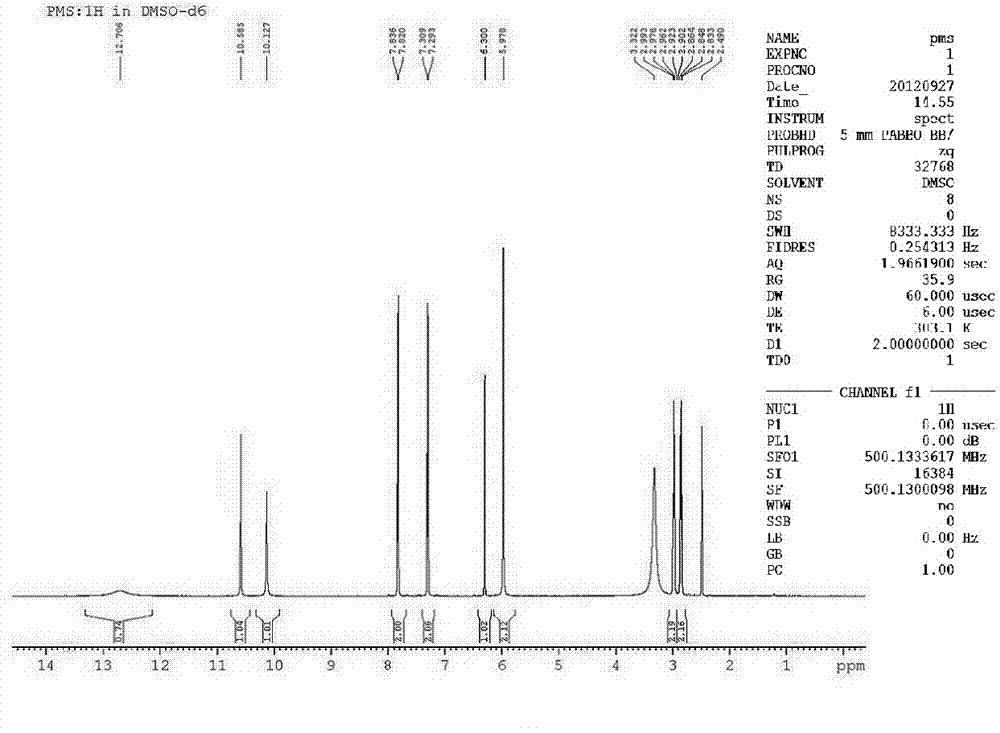
Image
Examples
Embodiment 1
[0058] 1. Preparation of 3-(4-methoxycarbonyl)phenyl-1-hydroxypropyl sodium sulfonate (A1)
[0059]
[0060] Under nitrogen protection, 220kg of dimethylformamide, 9.4kg of lithium acetate dihydrate, 10.6kg of lithium chloride, 18kg of methyl p-bromobenzoate, 6.8kg of propenol, and 5.4 kg of tetrabutylammonium bromide were added to a 500L reactor. kg. Raise the temperature to 85-90 degrees, then add 0.38 kg of palladium acetate, continue stirring for about 0.5 h, and monitor by TLC until the reaction is complete. After the reaction is complete, add the feed liquid to 330L water and extract twice with ethyl acetate, 150kg each time, combine the organic phases, wash once with 100kg water, and separate the organic phases; add 40kgNaHSO 3 Aqueous solution (containing NaHSO 3 8kg), stirred and crystallized to collect the solid, and dried at 45°C to obtain 22.4kg of light yellow solid (A1), with a yield of 90% and a purity (partially decomposed into aldehyde when detected by HP...
Embodiment 2
[0082] The following work is the scale-up production record of pemetacid prepared by the present inventors via route 1.
[0083] 1. Preparation of 3-(4-methoxycarbonyl)phenyl-1-propanal (B1)
[0084]
[0085] Under nitrogen protection, 220kg of DMF, 9.4kg of lithium acetate dihydrate, 10.6kg of lithium chloride, 18kg of methyl p-bromobenzoate, 6.8kg of propenol, and 5.4kg of tetrabutylammonium bromide were added to a 200L reactor. Raise the temperature to 85-90 degrees, then add 0.38 kg of palladium acetate, continue stirring for about 0.5 h, and monitor by TLC until the reaction is complete. After the reaction is complete, add the feed liquid to 330L of water, extract twice with ethyl acetate, 150kg each time, combine the organic phase, and wash once with 100kg of water, separate the organic phase; turn on the heating, control the temperature below 45 degrees to evaporate the solvent, 15kg of oil (B1) was obtained with a purity of 56% (22.5% impurity in 22min, MS analysi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com