Method for catalyzing gamma-valerolactone to be subjected to ring-opening polymerization by utilizing biomimetic catalyst
A technology of biomimetic catalyst and ring-opening polymerization, which is applied in the field of using biomimetic catalyst to catalyze the ring-opening polymerization of γ-valerolactone, can solve the problem of single substrate of polyester, and achieve high catalytic efficiency, wide sources and low dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
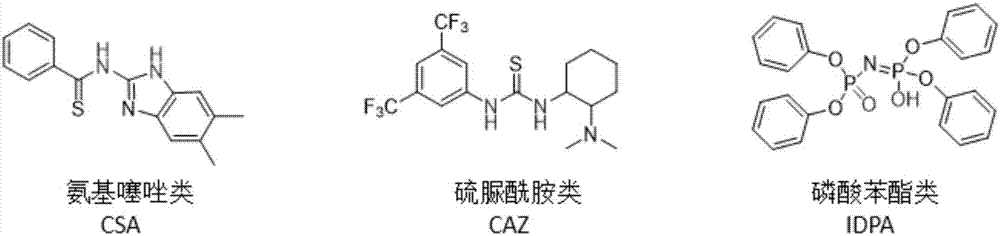
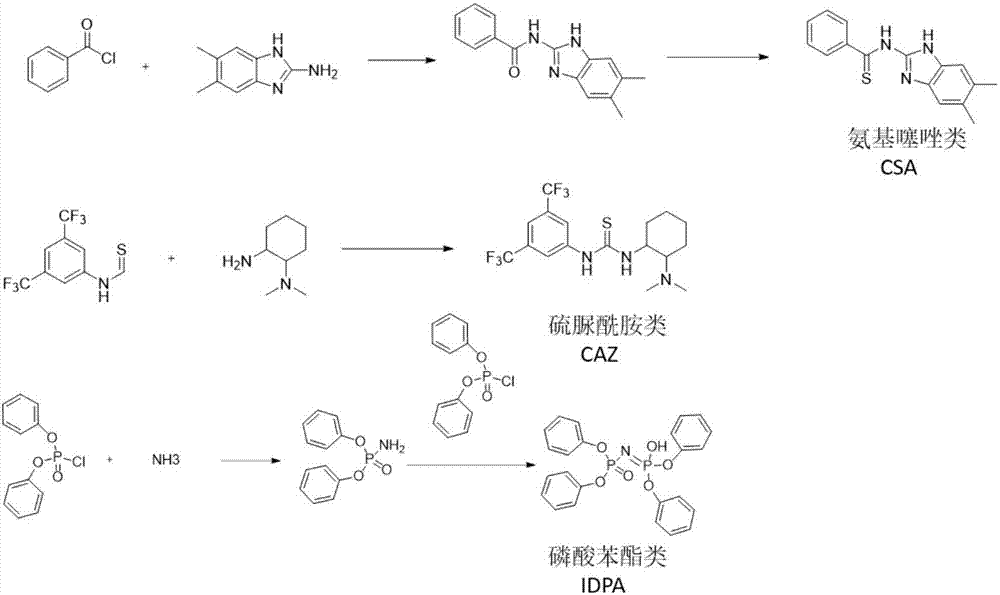
[0030] Preparation of aminothiazole-based biomimetic catalyst (CAZ): 3.3 mmol of benzoyl chloride was dissolved in 5 mL of THF, and 3 mmol of 2-amino-5,6-dimethylbenzimidazole and 5.7 mmol of N,N-diisopropyl were added dropwise The THF solution of ethylamine (15ml), the mixture was stirred at room temperature for 16h, after the reaction was completed, the reaction solution was evaporated to dryness, and the aminothiazole catalyst intermediate was obtained by column chromatography, and then 3mmol of the intermediate and 3mol of Lawson's reagent were taken, and 60ml of toluene was added as Solvent, under nitrogen protection, heating and refluxing for 16h, after the reaction, vacuum drying, column chromatography to obtain N-(5,6-dimethyl-1H-benzo[d]imidazol-2-yl)benzothiol amide.
Embodiment 2
[0032] Preparation of thiourea amide biomimetic catalyst (CSA): under argon protection, 1 mmol 3,5-bis(trifluoromethyl) phenyl isothiocyanate was dissolved in 1 ml of anhydrous THF, and N, N-dimethylcyclohexanediamine 2.23mmol, the reaction was stirred for 3h, the mixture was concentrated in vacuo, and the oily substance was purified by column chromatography to obtain -3,5-bis(trifluoromethyl)phenyl)-3-(2-(bis Methylamino) cyclohexyl) thiourea biomimetic catalyst.
Embodiment 3
[0034] Preparation of phenyl phosphate biomimetic catalyst pyrophosphoric acid catalyst (IDPA): firstly synthesize diphenyl ester phosphoramide, take 3 mmol of diphenyl chlorophosphate in a 50 ml flask in an anhydrous and oxygen-free environment, and pass it through it at -78 ° C Ammonia gas was added to react for 2 h. After the reaction, the cooling bath was removed, the product was vacuum-dried, and the product was purified by column chromatography (dichloromethane was used as the eluent). Get 2mmol diphenyl ester phosphoramide and 2mmol diphenyl chlorophosphate and 50ml flask, add 5ml THF to it, add 5mmol 60% sodium hydroxide solution to it in addition, stir at room temperature for 14h, add 10ml 10% after the reaction finishes Hydrochloric acid and 10 ml of dichloromethane (DCM), stirred for 1 h, the organic phase was taken out, and the product was purified by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com