Thermostable carboxylesterase gene, coding protein and application thereof
A carboxylesterase, thermostable technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of less active protein, lack of post-translational modification system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Acquisition of carboxylesterase gene estW
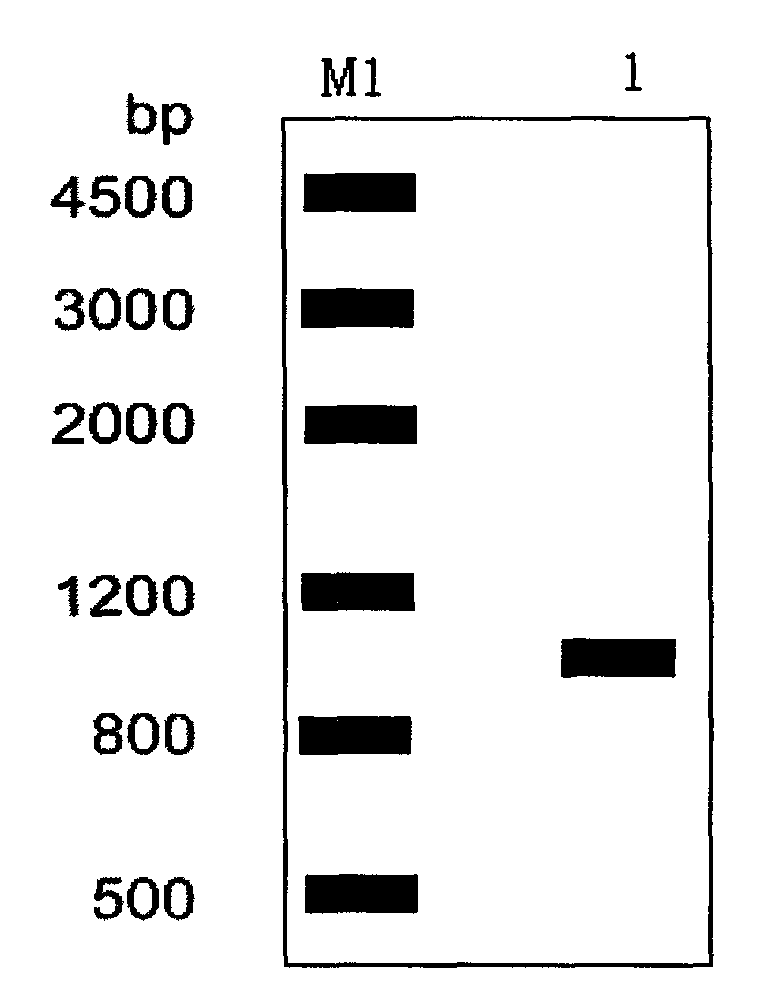
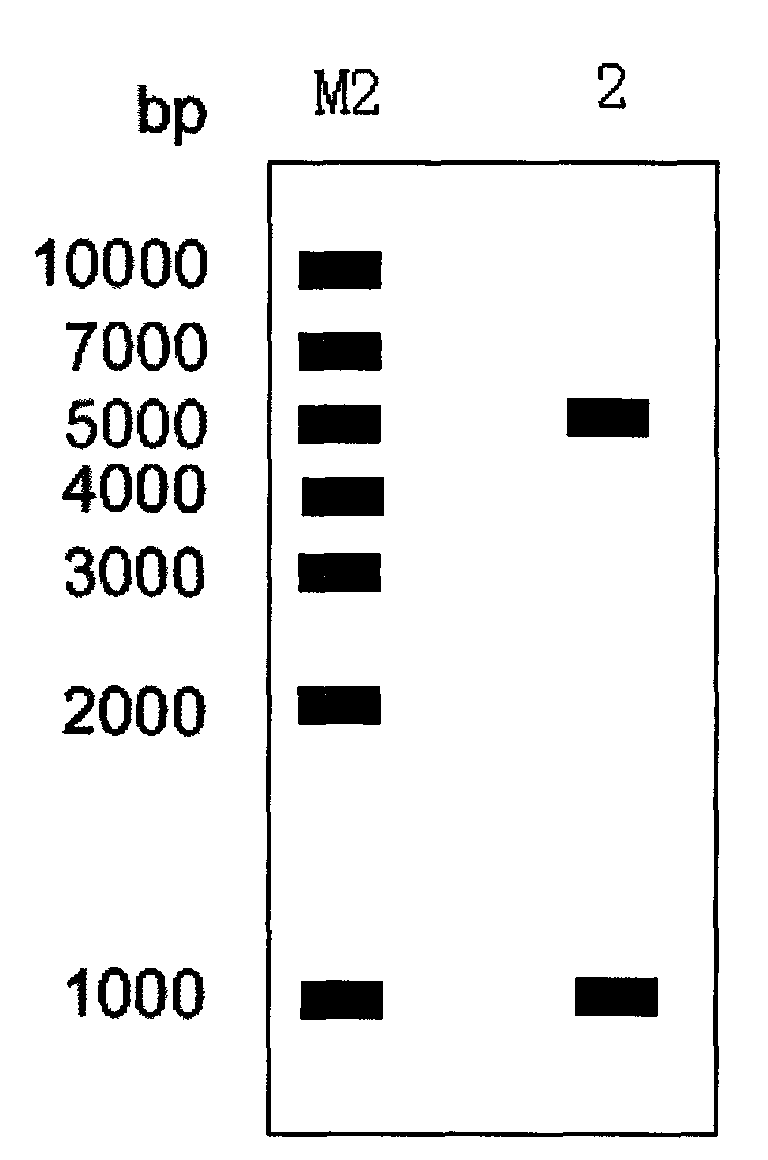
[0023] Cultivate Streptomyces lividans TK64 and extract the genome, use its genomic DNA as a template, and use estW-F: ATACGTC Catatgag TTTCCTCAATCCCCGCAT and estW-R:TAA CTCGAG CGATTATAAAGCTTCCCG (the underlines are NdeI and XhoI restriction sites respectively) as primers, PCR amplification was carried out under the conditions of 98°C, 10s, 55°C, 10s, 72°C, 1min 10s, 35 cycles, and the products were agarose gel Electrophoresis ( figure 1 ), subsequently, the amplified product and the vector pET28b(+) were digested by NdeI and XhoI respectively, then ligated, transformed into E. cut identification ( figure 2 ); the expression plasmid pET28b-estW is sequenced, and the amino acid sequence is shown in SEQ ID NO:1.
[0024] 2. Construction of expression engineering bacteria
[0025] The expression plasmid pET28b-estW was transformed into Escherichia coli Rosetta (DE3) competent cells according to the existing technology,...
Embodiment 2
[0031] EstW optimum temperature detection:
[0032] EstW enzyme activity detection standard system 1ml, 50mM Tris-HCL (pH 8.0), 1% acetonitrile, 0.5mM p-nitrophenol hexanoate, 5μl pure enzyme solution, using a Cary 300 UV spectrophotometer with automatic temperature control , the detection wavelength is 410nm. The reaction temperature (10°C-60°C) was set, the test was repeated 3 times independently, and the average value was taken. The results showed that the optimum temperature of the enzyme was 50°C ( Figure 4 ).
Embodiment 3
[0034] EstW optimal pH detection
[0035]Using a standard assay system, the reaction temperature is 50°C, and the corresponding buffer solution is used in the corresponding pH range, pH5.0 to 7.04, 50mM sodium citrate buffer; pH 7.0-9.0, 50mM Tris·HCl buffer; pH 8.5-9.0, 50mM K 2 HPO 4 -KOH buffer. Detection wavelength is OD 348 , the experiment was repeated three times independently, and the results showed that the optimum pH of the enzyme was 8.0 ( Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com