Enteric sustained-release preparation containing zaltoprofen and preparation method thereof
A technology for zaltoprofen and sustained-release preparations, which is applied in the field of enteric-coated sustained-release preparations and its preparation, and can solve problems such as poor reproducibility of release between batches, poor fluidity, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
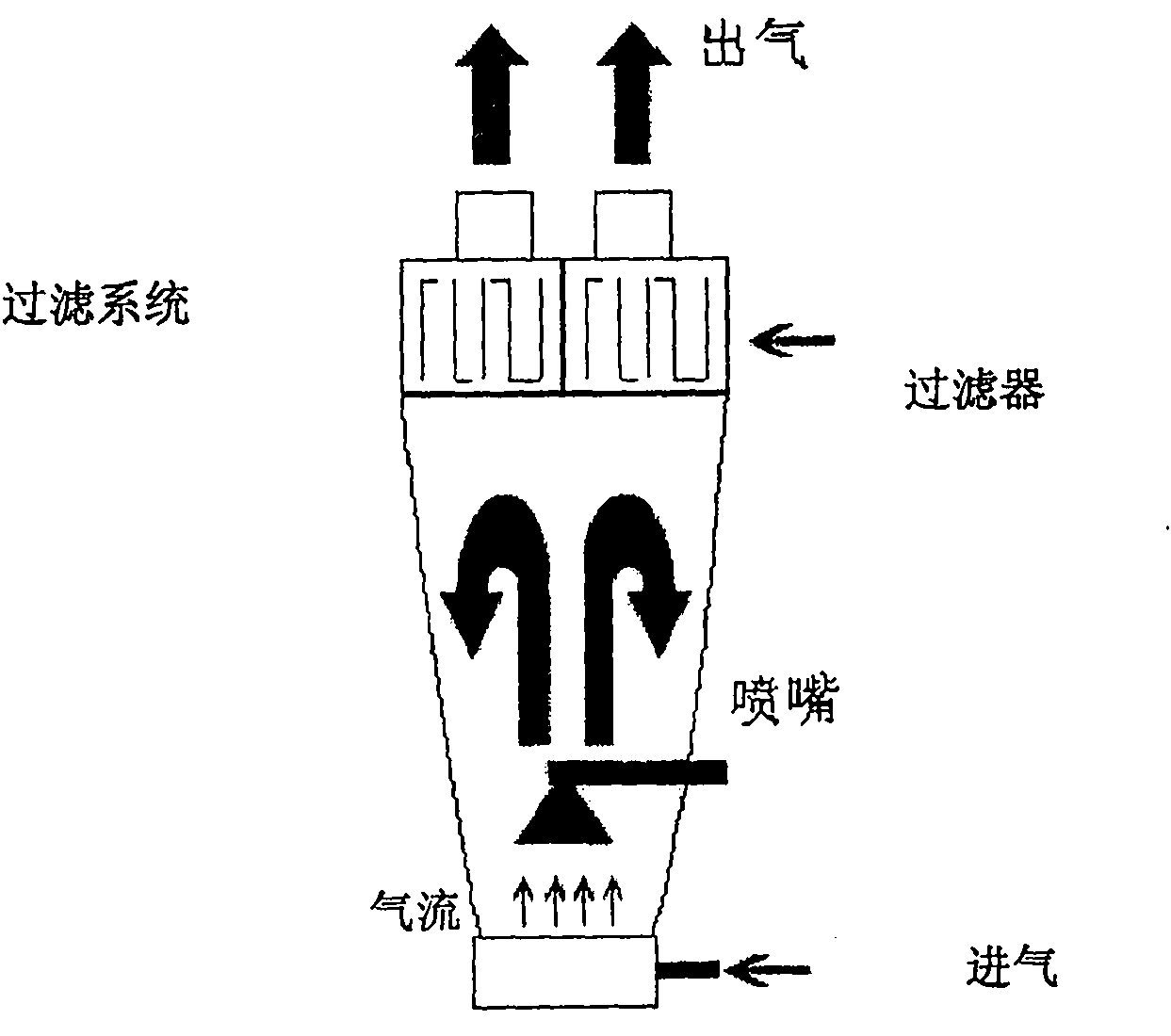
Image
Examples
Embodiment 1
[0050] Zaltoprofen crystal (particle size 200-300μm, spherical crystal) 200g
[0051]Glyceryl Behenate 10g
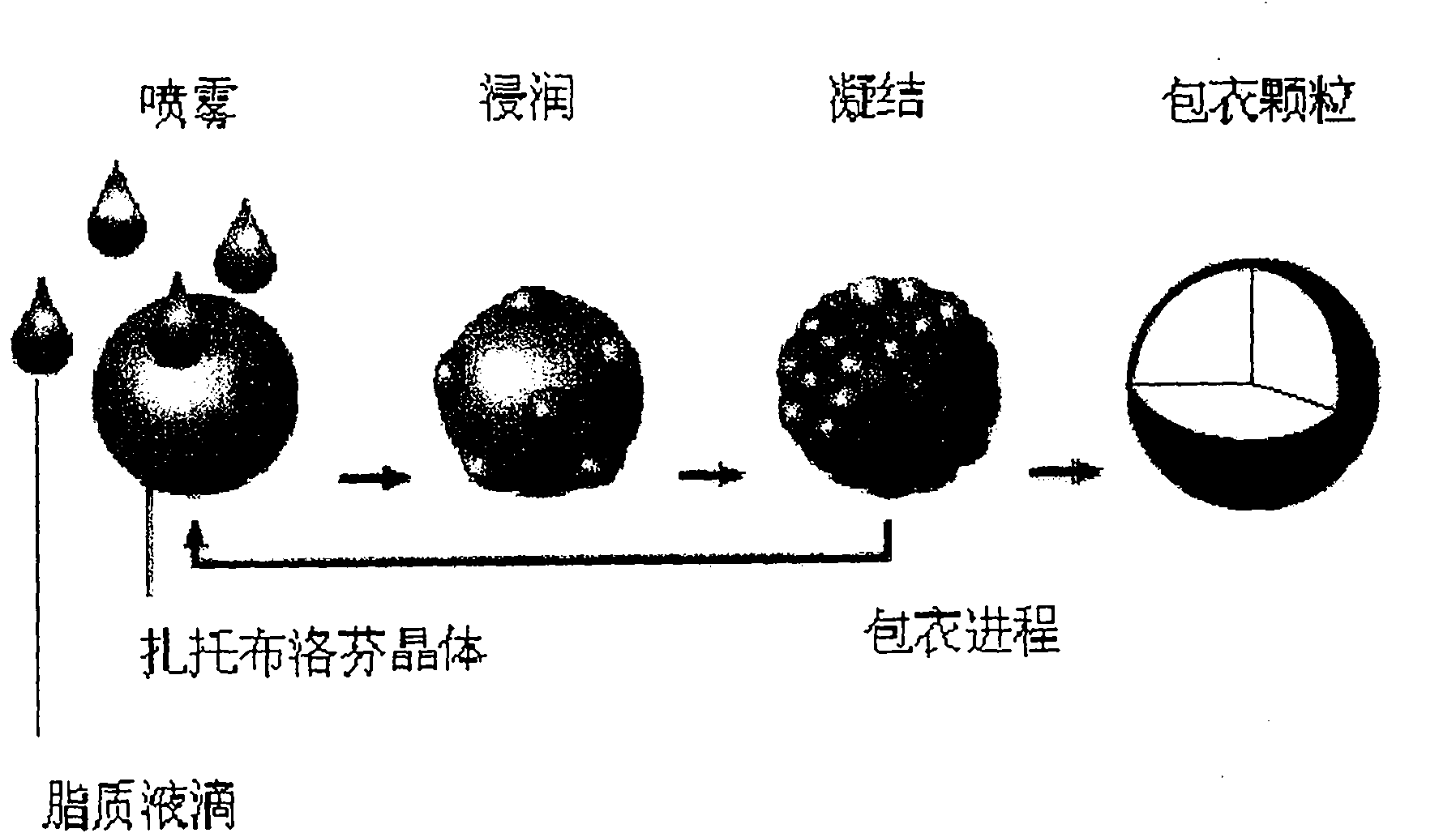
[0052] Precisely weigh 200g of Zaltobuprofen spherical crystals, place them in a fluidized bed for boiling at a constant temperature, and control the inlet temperature at about 50°C; take glyceryl behenate and heat and melt it, and finally keep the temperature at 85°C; The glyceride liquid is introduced into the fluidized bed, atomized by high-pressure air, and top spray technology is used to coat the liquid atomized glyceryl behenate droplets on the surface of the fluidized tobuprofen crystals in the fluidized bed. Since the crystal temperature is lower than the melting point of glyceryl behenate, when the liquid atomized glyceryl behenate droplets meet zaltoprofen crystals, they will quickly condense into tiny solid particles and be coated with zaltoprofen On the surface of the particles, the spray speed of glyceryl behenate needs to be controlled so that the tiny so...
Embodiment 2
[0055] Zaltoprofen crystal (particle size 200-300μm, spherical crystal) 200g
[0056] Glyceryl Behenate 20g
[0057] Precisely weigh 200g of Zaltobuprofen spherical crystals, place them in a fluidized bed for boiling at a constant temperature, and control the inlet temperature at about 50°C; take glyceryl behenate and heat and melt it, and finally keep the temperature at 85°C; Glyceride liquid is introduced in the fluidized bed, adopts high-pressure air atomization (atomization pressure is the same as embodiment 1), adopts top spray technology, makes liquid atomization glyceryl behenate small droplet be coated on fluidized bed On the surface of zaltoprofen crystals, since the crystal temperature is lower than the melting point of glyceryl behenate, the small droplets of liquid atomized glyceryl behenate will quickly condense into tiny solid particles after meeting zaltoprofen crystals And be coated on zaltoprofen crystal surface, need control the spray speed of glyceryl behen...
Embodiment 3
[0060] Zaltoprofen crystal (particle size 200-300μm, spherical crystal) 200g
[0061] Glyceryl Behenate 30g
[0062] Precisely weigh 200g of Zaltobuprofen spherical crystals, place them in a fluidized bed for boiling at a constant temperature, and control the inlet temperature at about 50°C; take glyceryl behenate and heat and melt it, and finally keep the temperature at 85°C; Glyceride liquid is introduced in the fluidized bed, adopts high-pressure air atomization (atomization pressure is the same as embodiment 1), adopts top spray technology, makes liquid atomization glyceryl behenate small droplet be coated on fluidized bed On the surface of zaltoprofen crystals, since the crystal temperature is lower than the melting point of glyceryl behenate, the small droplets of liquid atomized glyceryl behenate will quickly condense into tiny solid particles after meeting zaltoprofen crystals And be coated on zaltoprofen crystal surface, need control the spray speed of glyceryl behen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com