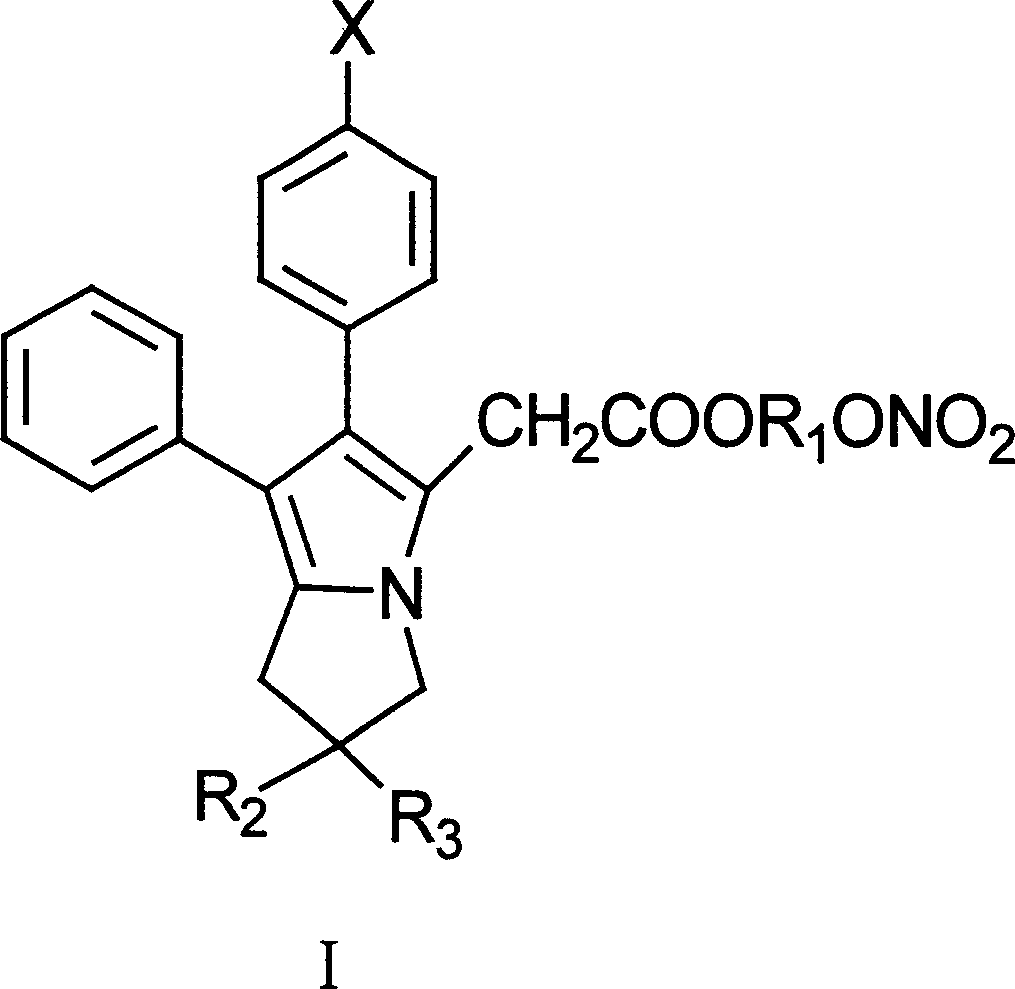
Antipyretic analgesic and anti-arthritis nonsteroidal compound and its pharmaceutical compositions
A compound and composition technology, applied in the field of new compounds and their preparation, can solve the problems of inability to block the generation of metabolic pathway products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
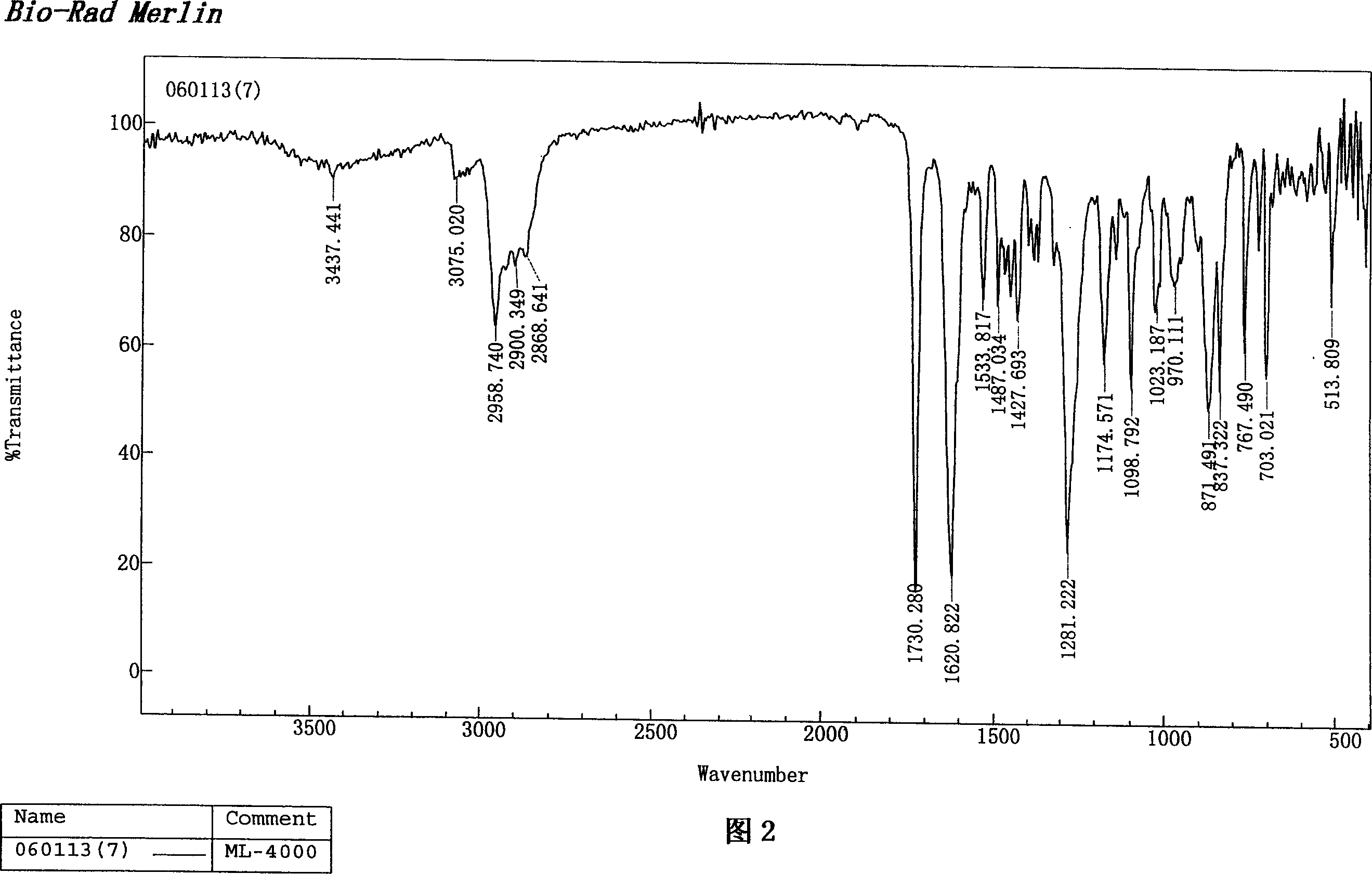
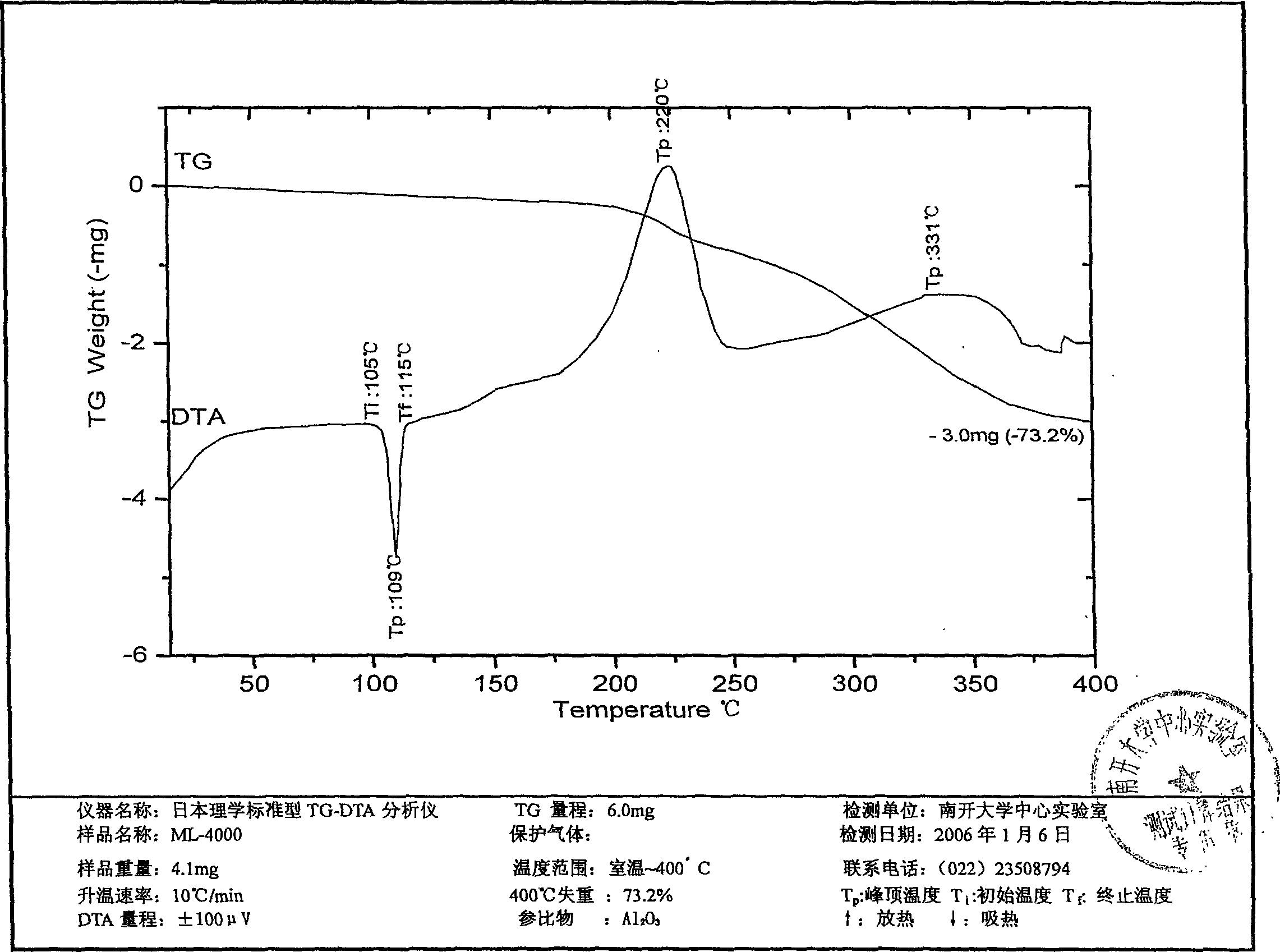
Image
Examples
Embodiment 1
[0063] Preparation of 6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-pyrrolizine-5-bromobutyl acetate (v):
[0064] Put 50 milliliters of analytically pure methanol into a 250 milliliter four-neck reaction flask, stir and reflux, add 1.5 grams of sodium metal, reflux for 10 minutes, and cool down to room temperature; add 7.5 grams (0.02mol) of 6-(4-chlorophenyl) -2,2-Dimethyl-7-phenyl-2,3-dihydro-pyrrolizine-5-acetic acid (IV), reacted for 30 minutes; distilled to dryness under reduced pressure at 50-60°C, added 50 ml di Methylformamide and 12.9 g (0.06 mol) redistilled 1,4-dibromobutane were reacted at room temperature for 24 hours, then distilled to dryness under reduced pressure at 100°C to obtain an oil. Using 200-mesh silica gel column analysis, using n-hexane and diethyl ether at a ratio of 1:1 as the developing solvent, 7-8 grams of light yellow solid (v) was obtained with a yield of 60-70% and a melting point of 89-92°C.
[0065] HNMR (CDCl 3 )δ: 1.29 (6H, two ...
Embodiment 2
[0067] Preparation of 6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-pyrrolizine-5-acetic acid butyl nitrate (II):
[0068] In a 250 ml four-neck reaction, 50 ml of tetrahydrofuran and 8 g (0.015 mol) of (v) were added at room temperature, and stirred to dissolve. 4 grams (0.023mol) of silver nitrate were dissolved in 30 milliliters of acetonitrile, and added to a 250 milliliter four-neck reaction flask. After reacting at room temperature for 24 hours, 1 gram (0.006mol) of silver nitrate was added to continue the reaction at room temperature for 24 hours, 50- Distilled to dryness under reduced pressure at 60°C to obtain oil. Using 200-mesh silica gel column analysis, using n-hexane and diethyl ether as a developing solvent at a ratio of 7:3, 4 g of (II) was obtained with a yield of 40% and a melting point of 105-107°C.
[0069] HNMR (CDCl 3 )δ: 1.30 (6H, two -CH at the 2-position of the pyrrolizine ring 3 Hydrogen), 1.76 (4H, two -CH in the 2'.3' position of butyl nit...
Embodiment 3
[0071] Preparation of 6-(4-bromophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-pyrrolizine-5-bromopropyl acetate (III):
[0072] Put 50 milliliters of analytically pure methanol into a 250 milliliter four-neck reaction flask, stir and reflux, add 1.5 grams of sodium metal, reflux for 10 minutes, and cool down to room temperature; add 8.5 grams (0.02mol) of 6-(4-bromophenyl) -2,2-Dimethyl-7-phenyl-2,3-dihydro-pyrrolizine-5-acetic acid (VI), reacted for 30 minutes; distilled to dryness under reduced pressure at 50-60°C, added 50 ml di Methylformamide and 12 g (0.06 mol) of redistilled 1,4-dibromopropane were reacted at room temperature for 24 hours, then distilled to dryness under reduced pressure at 100°C to obtain an oil. Using 200-mesh silica gel column analysis, using n-hexane and diethyl ether at a ratio of 1:1 as the developer, 6 g of a light yellow solid was obtained with a yield of 70%. The crude product was directly put into the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com