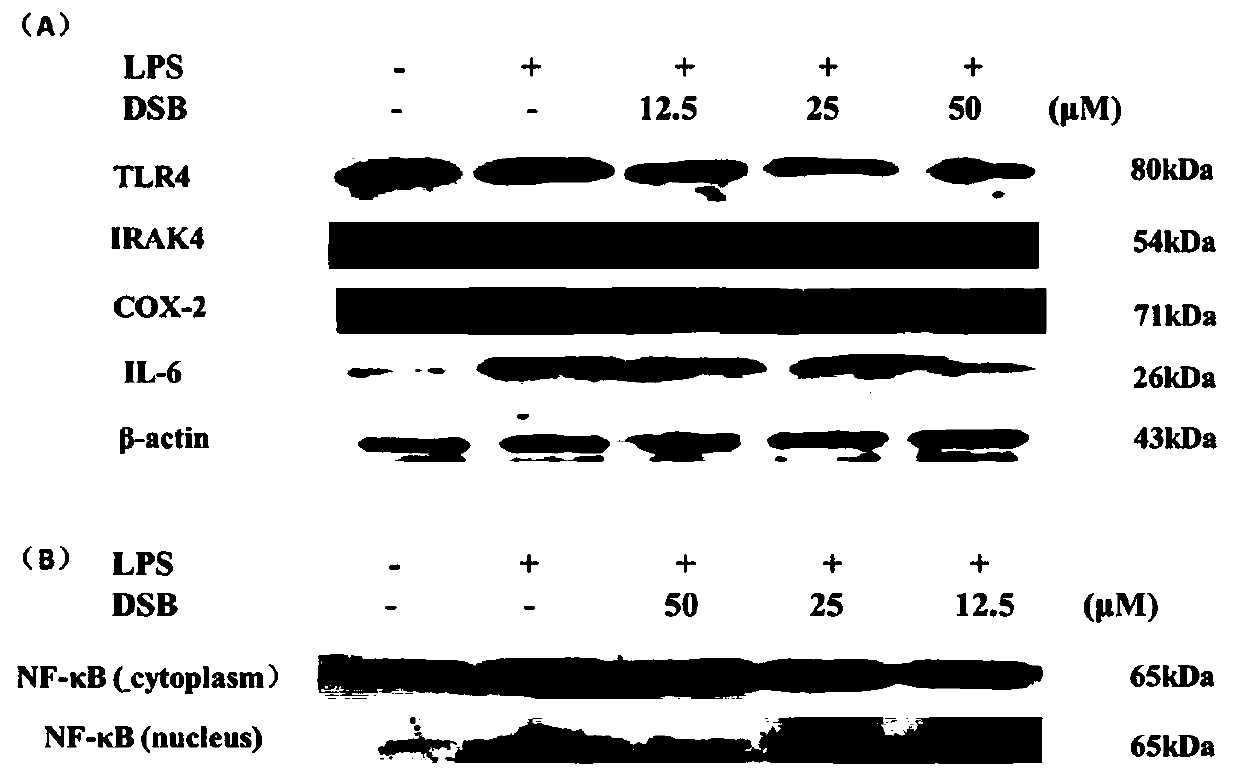
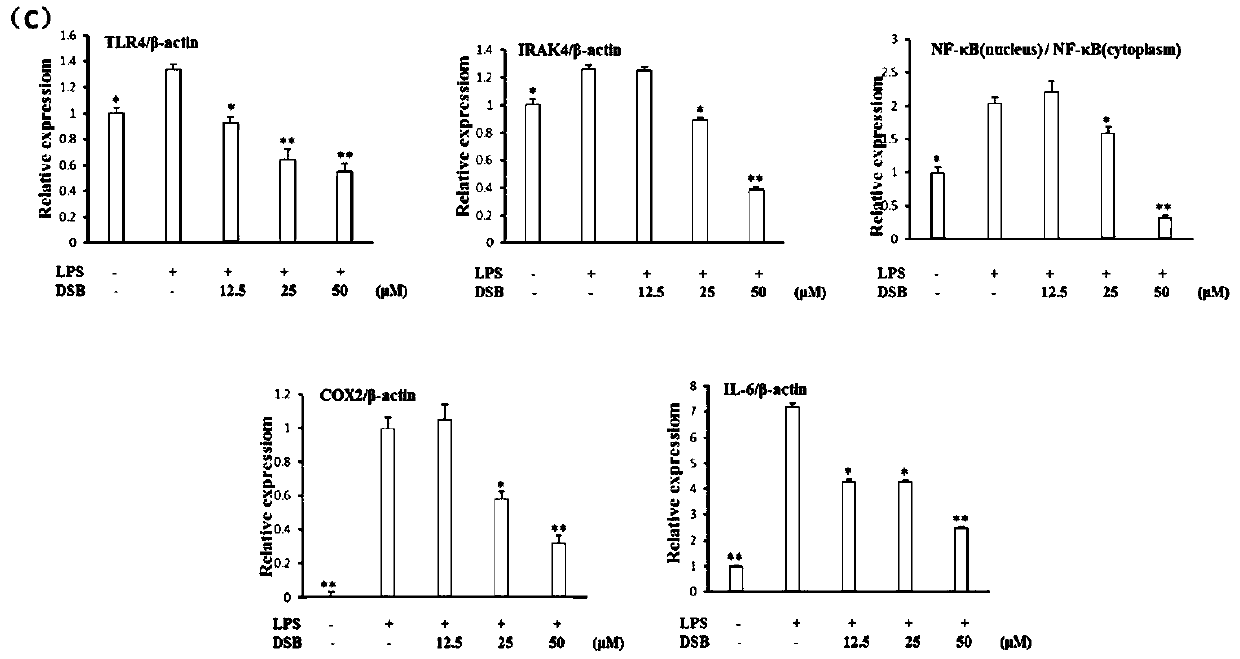
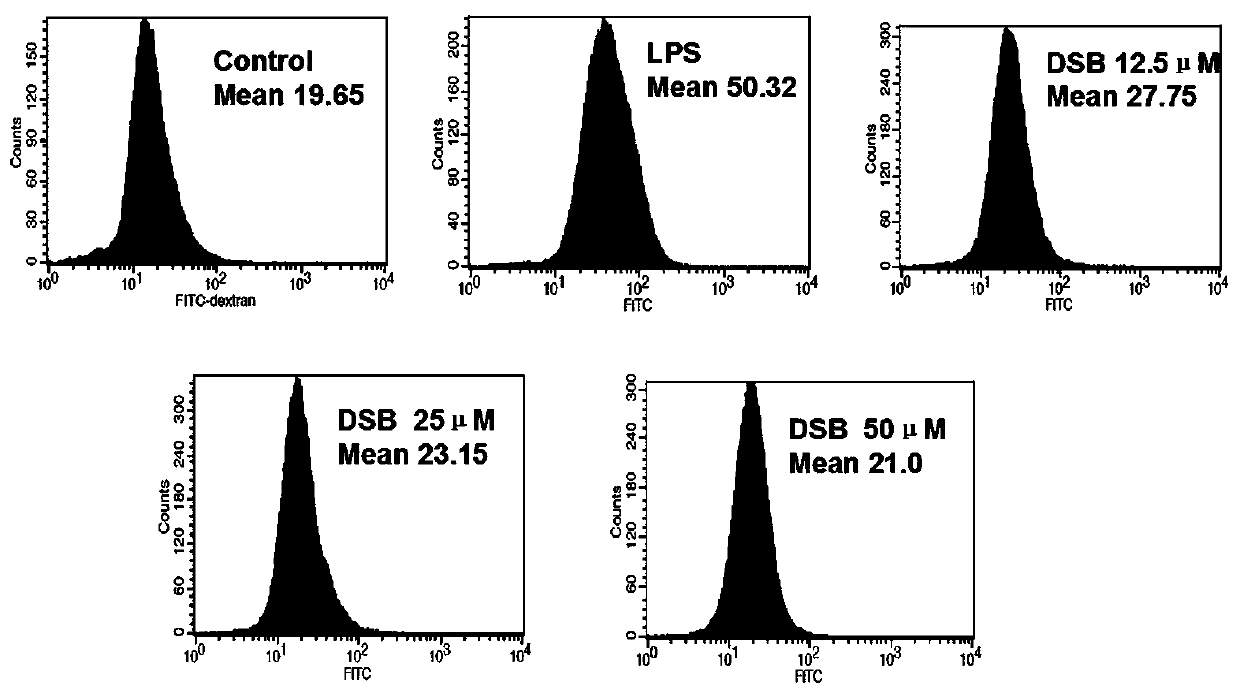
tlr4/md2 inhibitors and their application in anti-inflammatory drugs
A technology of anti-inflammatory drugs and inhibitors, which is applied in the field of new TLR4/MD2 inhibitors, can solve the problems of unreported structure and pharmacological activity, etc., and achieve clear anti-inflammatory effects, inhibit release, and strong effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation and structural confirmation of compound 4-(5-dimethylamino-1-naphthalene)sulfonyloxybenzoxazol-2-one (DSB)
[0045] Weigh 500 mg of the compound 4-hydroxybenzoxazolone and dissolve it in 30 mL of anhydrous acetone, gradually add 500 μL of acid-binding agent triethylamine dropwise, react in an ice bath for 30 minutes, and slowly add 5-dimethylamino-1-naphthalene dropwise 20 mL of anhydrous acetone solution of 900 mg of sulfonyl chloride, reacted at room temperature for 30 min, then refluxed at 55°C for 6 h, evaporated the solvent, and separated by column chromatography to obtain the target compound 4-(5-dimethylamino-1-naphthalene)sulfonyl Oxybenzoxazol-2-one (DSB) 700mg.
[0046] Structural characterization: molecular formula C 19 h 16 N 2 o 5 S (384.08g / mol); Yellow powder; Yield (%): 65.3%; mp (°C): 164.3-166.4°C; ESI-MS (m / z): 385.17 ([M+H]+); 1 H NMR (600MHz, CDCl 3 ):δ3.05(s,6H,CH 3 ), 6.44(d, J=8.4Hz, 1H, Ar-5-H), 6.83(t, 1H, J=8.4Hz,...
Embodiment 2
[0047] Example 2: Preparation and structural confirmation of compound N-benzyl-4-(5-dimethylamino-1-naphthalene)sulfonyloxybenzoxazol-2-one (DSB-2)
[0048] Weigh 50 mg of 4-(5-dimethylamino-1-naphthalenesulfonyloxy)benzoxazol-2-one (DSB) in a 50 mL eggplant-shaped bottle, dissolve in 20 mL of anhydrous acetone, add 96.9 mg of Water K 2 CO 3 In the reaction system, after reacting at room temperature for 30 minutes, add twice the equivalent of benzyl bromide, reflux at 60° C. for 6 hours, and monitor the end point of the reaction by TLC. Filter to remove solid K 2 CO 3 , spin to dry the solvent, take ethyl acetate / petroleum ether (1:9) as the eluent system, separate and purify by column chromatography to obtain the target compound N-benzyl-4-(5-dimethylamino-1-naphthalene)sulfonyl Oxybenzoxazol-2-one (DSB-2).
[0049] Structural characterization: molecular formula C 26 h 22 N 2 SO 5 , the yield is 51.40%; mp.162.0~164.8℃; the property is bright yellow solid powder; 1 ...
Embodiment 3
[0050] Example 3: Preparation and structural confirmation of the compound N-phenethyl-4-(5-dimethylamino-1-naphthalene)sulfonyloxybenzoxazol-2-one (DSB-3)
[0051] Weigh 100mg of 4-(5-dimethylamino-1-naphthalenesulfonyloxy)benzoxazol-2-one (DSB) into a 50mL eggplant-shaped bottle, dissolve it in 30mL of anhydrous acetone, add 120mg of anhydrous K 2 CO 3 In the reaction system, after reacting at room temperature for 45 minutes, add twice the equivalent of phenethyl bromide, reflux at 65° C. for 8 hours, and monitor the end point of the reaction by TLC. Filter to remove solid K 2 CO 3 , spin to dry the solvent, take ethyl acetate / petroleum ether (2:8) as the eluent system, separate and purify by column chromatography to obtain the target compound N-phenethyl-4-(5-dimethylamino-1-naphthalene)sulfonate Acyloxybenzoxazol-2-one (DSB-3).
[0052] Structural characterization: molecular formula C 27 h 24 N 2 SO 5 , yield 39.03%; mp.141.8~145.3°C; property is yellow-green solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com