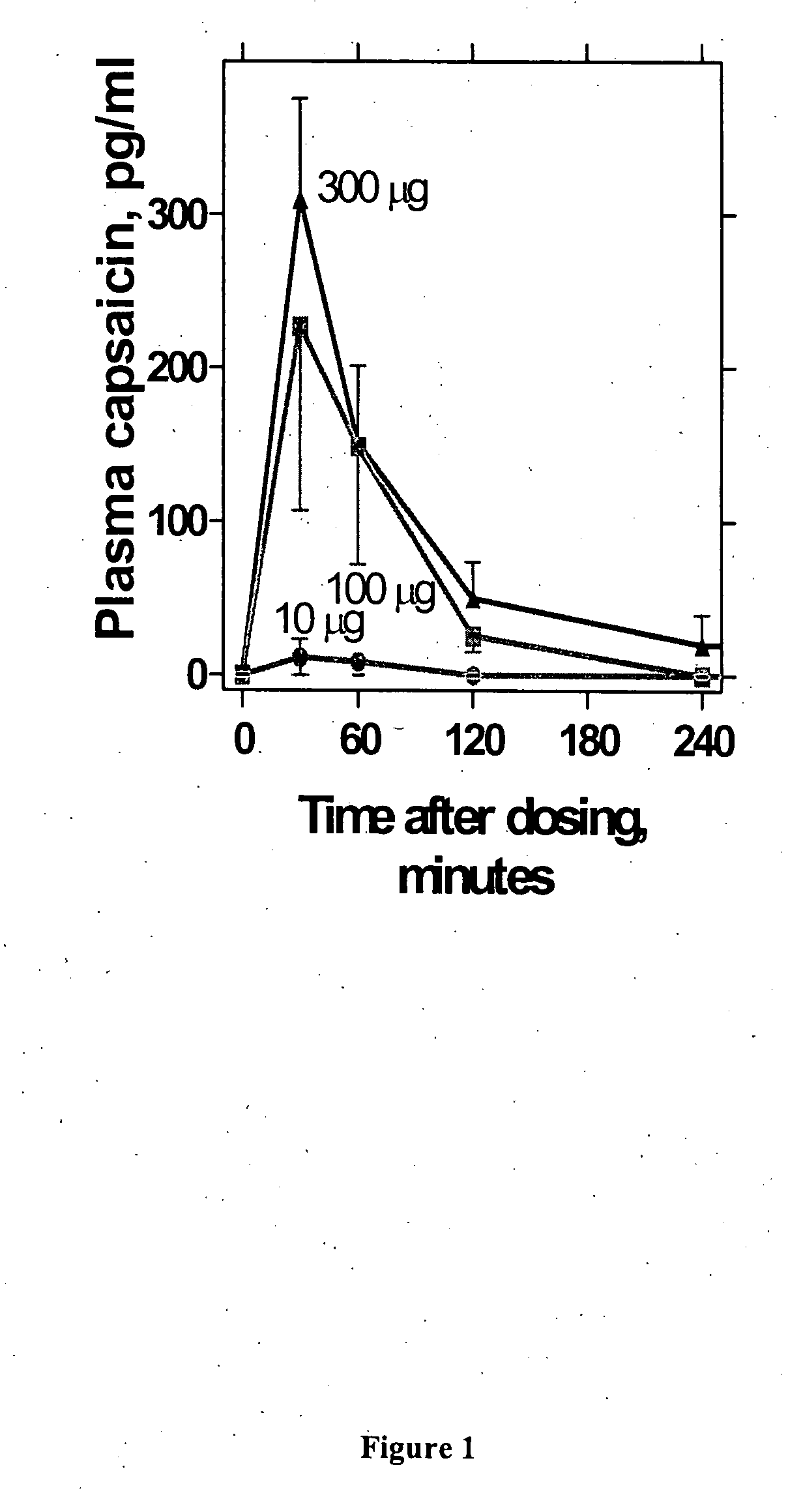
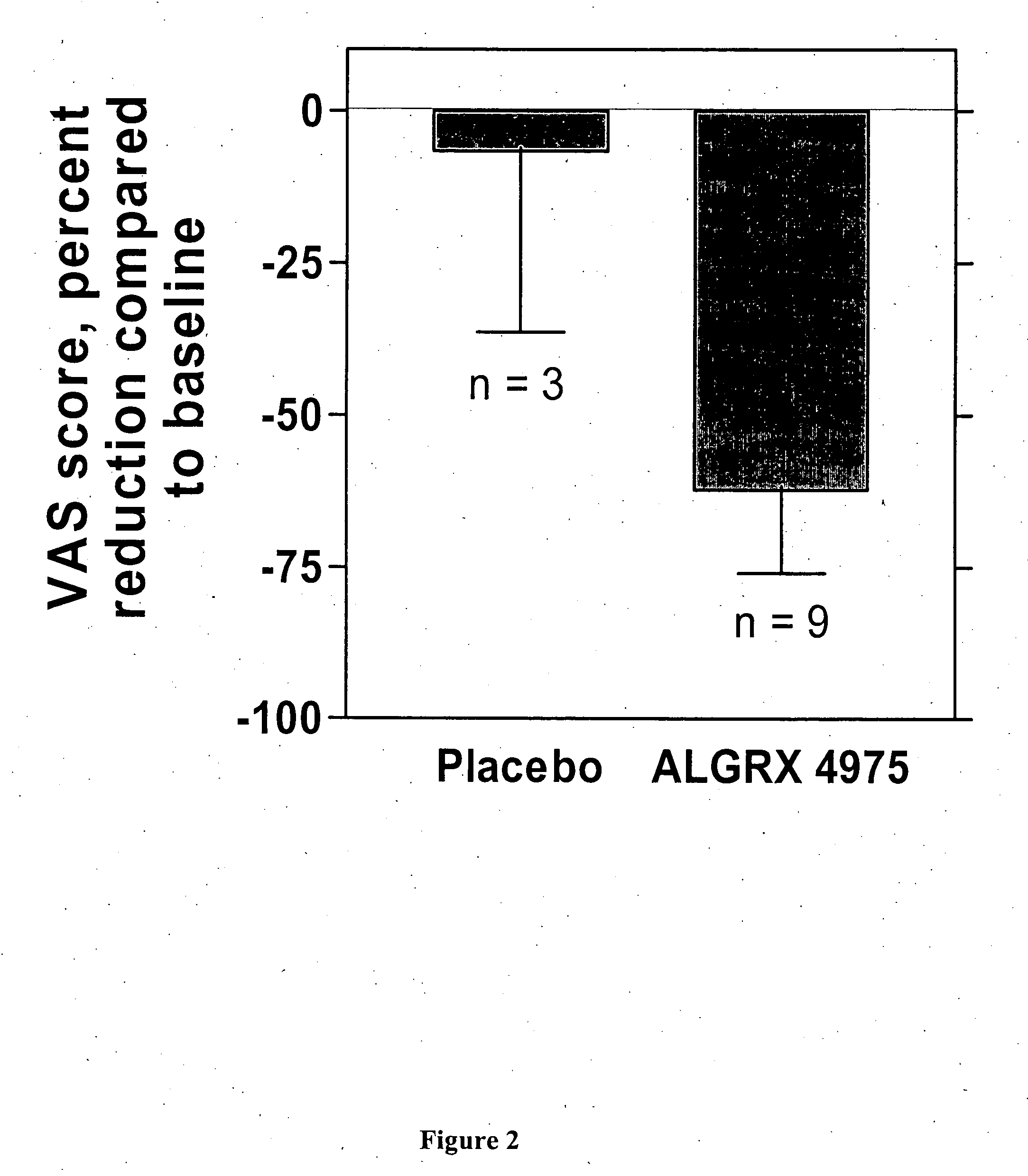
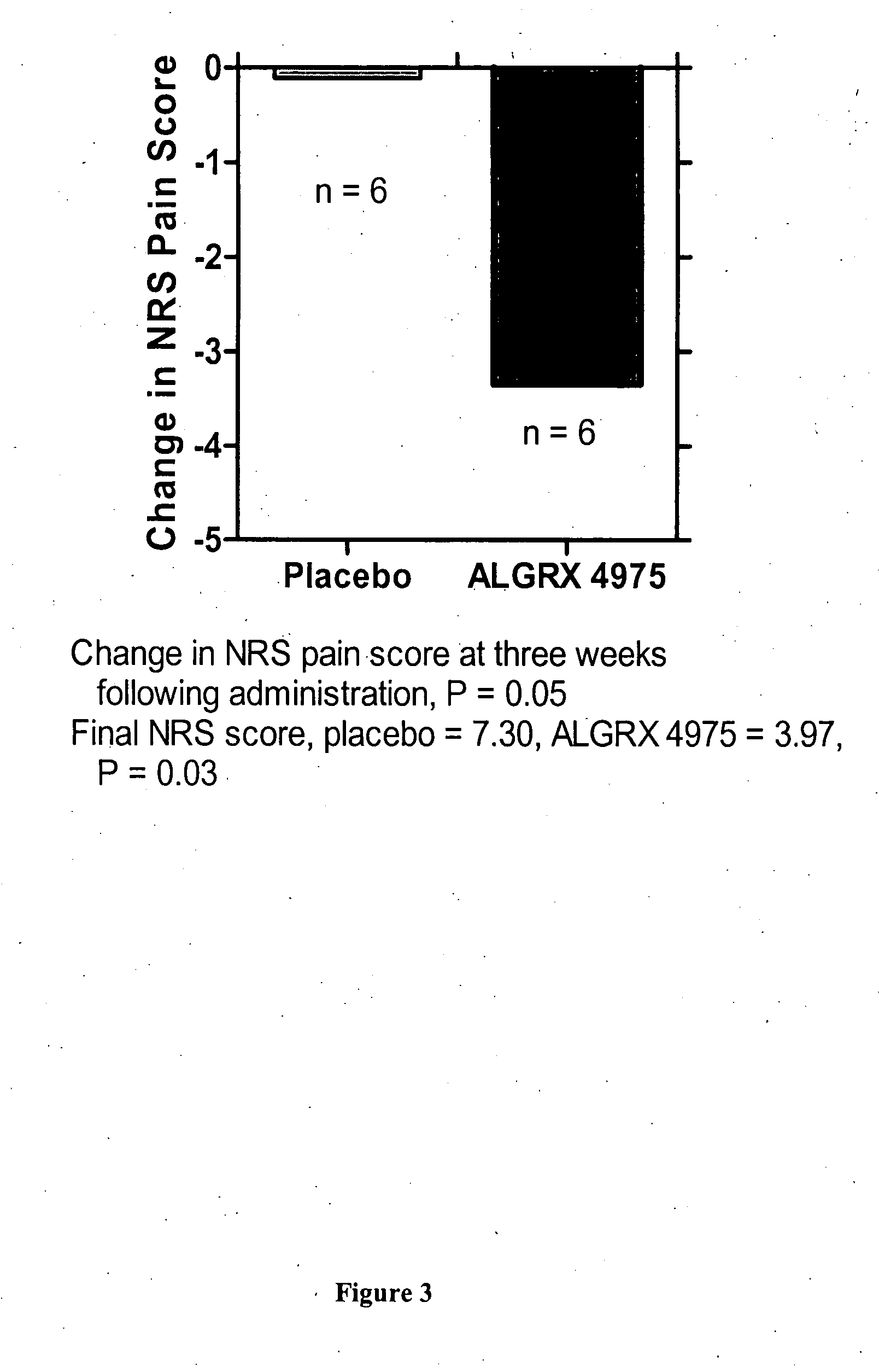
Administration of capsaicinoids
a technology of capsaicin and capsaicin, which is applied in the direction of biocide, plant/algae/fungi/lichens, biocide composition, etc., can solve the problems of poor patient compliance, high dropout rate during clinical trials, and limited effectiveness of topically administered capsaicin in general. , to achieve the effect of reducing or eliminating such side effects, improving movement, and alleviating pain at the si
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example ii
Osteoarthritis of the Knee Efficacy Study
[0252] The following clinical study evaluates the efficacy of purified capsaicin administered by intra-articular infiltration together with a local anesthetic injected by intra-articular infiltration in subjects with osteoarthritis of the knee.
[0253] The primary objective of the study is to evaluate the efficacy of intra-articular capsaicin, when co-administered with intra-articular local anesthetic, compared to placebo, in subjects with end-stage osteoarthritis of the knee, already scheduled to receive knee replacements (21 and 42 days after injection of study medication).
[0254] Purified capsaicin is supplied in vials containing 5 mL of purified capsaicin at a concentrations of 500 .mu.g / mL. Study drug was stored at a temperature between 15.degree. C. and 25.degree. C. Within four hours prior to injection, vehicle is used to dilute the drug to final concentrations of purified capsaicin, as follows:
15TABLE 2 Dose Level Concentration Total Vol...
example iii
Bunionectomy Efficacy Study
[0261] The following study was carried out in order to evaluate the safety, tolerability, systemic pharmacokinetics, and efficacy of intra-operative (infiltration) capsaicin when co-administered with a local anesthetic in patients scheduled to undergo transpositional osteotomy (bunionectomy).
[0262] The primary objective of the study was to evaluate the safety and tolerability of capsaicin, when co-administered by intra-articular infiltration with a local anesthetic, compared to placebo, in subjects with hallux valgus deformity, already scheduled to undergo transpositional osteotomy (bunionectomy). The secondary objective of the study was to evaluate the safety, tolerability and systemic pharmacokinetics of purified capsaicin following intra-operative administration. The primary efficacy endpoint was the proportion of subjects in each treatment group requiring opioid analgesia in the first 24 hours post-operatively. The proportions were compared amongst tre...
example iv
Median Sternotomy Study
[0277] The primary objective of the study is to determine the amount of opioid consumption and postoperative pain scores following median stemotomy for patients receiving purified capsaicin by infiltration and / or injection. Eligible subjects are patients undergoing cardiac, pulmonary, or mediastinal surgery for any indication between the ages of 20-70 years. The operation is performed under general anesthesia and are closely observed in a post-anesthesia care unit as per the practice of the institution. The study drug will be administered to the sternal edges, muscles (e.g., muscle edges), bone (e.g., bone edges), and tissues. All patients receive standard of care opioid on demand for treatment of pain when transferred to the ward. The dose of capsaicin is administered to the sternal edges, the muscle, the tissues and / or bone.
[0278] Pain is assessed utilizing VAS 100 mm scale--baseline, every 60 minutes beginning when the patient first is placed in a bedside c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com