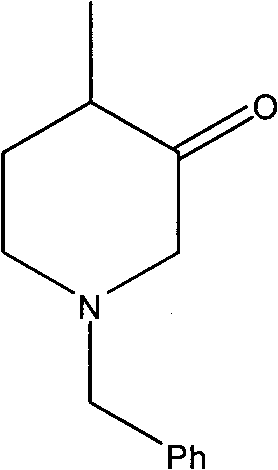
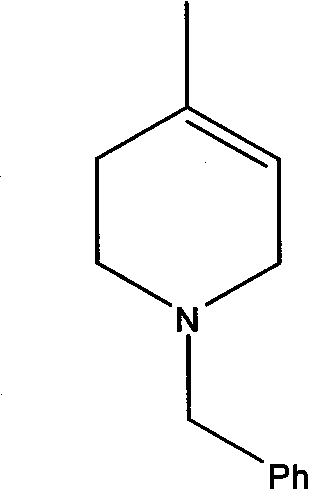
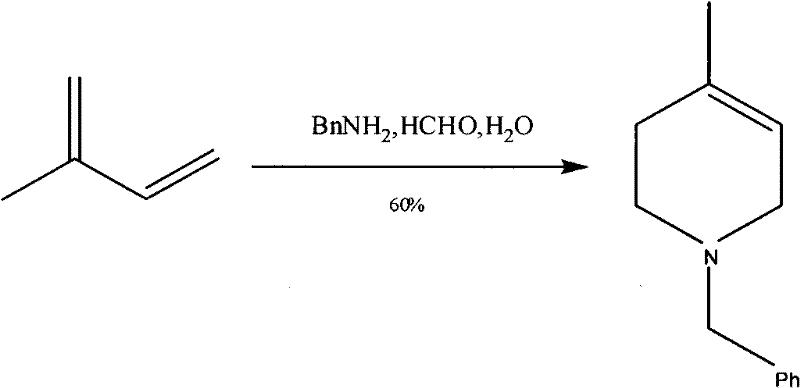
The synthetic method of n-benzyl-4-methyl-3-piperidone
A synthetic method, the technology of methylpiperidine, which is applied in the field of synthesis of organic compound N-benzyl-4-methyl-3-piperidone, can solve the problems of reduced yield, complex and harsh oxidation reaction operation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, a kind of synthetic method of N-benzyl-4-methyl-3-piperidone, with 3-hydroxyl-4-picoline and benzyl chloride as starting raw materials, the following steps are carried out successively:
[0045] 1) Synthesis of the benzyl chloride salt (compound 1) of 3-hydroxyl-4-picoline
[0046] Add 6.0g (0.055mol) of 3-hydroxy-4-methylpyridine to 80ml of acetonitrile, and dropwise add an acetonitrile solution of benzyl chloride (8.5g (0.067mol) of benzyl chloride dissolved in 10ml of acetonitrile) at room temperature, and the addition is complete Finally, continue to stir at room temperature for 15 minutes, then heat up to reflux, during the heating process, the reaction system gradually becomes a homogeneous system, and the reflux reaction is overnight, and the reaction process is detected by TLC (ethyl acetate / methanol=10: 1); the reaction is complete After cooling to room temperature, 4 / 5 of the acetonitrile was distilled off under reduced pressure, and 100ml of eth...
Embodiment 2
[0061] Embodiment 2, a kind of synthetic method of N-benzyl 4-methyl-3-piperidone,
[0062] In step 1): the molar ratio of benzyl chloride and 3-hydroxyl-4-picoline is adjusted to 1.05: 1 (the addition of benzyl chloride is 7.4g (0.058mol)), and other operations are the same as in Example 1 to obtain the first The yield of the one-step target product compound 1 is 90.84%, and the purity is 95.36%.
[0063] Step 2) and step 3) are the same as in Example 1; the total yield is 86.1%.
Embodiment 3
[0064] Embodiment 3, a kind of synthetic method of N-benzyl-4-methyl-3-piperidone,
[0065] In step 1):
[0066] The reaction solvent I was tetrahydrofuran (replacing the acetonitrile in Example 1), and the other operations were the same as in Example 1. The yield of the target product Compound 1 in the first step was 88.42%, and the purity was 94.55%.
[0067] Step 2) and step 3) are the same as in Example 1; the total yield is 83.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com