Triaryldimethylpiperazine dihydrochloride polymorphic substance, preparation method and application thereof
A technology of dimethylpiperazine and ketone dihydrochloride, applied in the field of medicine, can solve problems such as inability to concentrate, sexual dysfunction, confusion, and narcolepsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
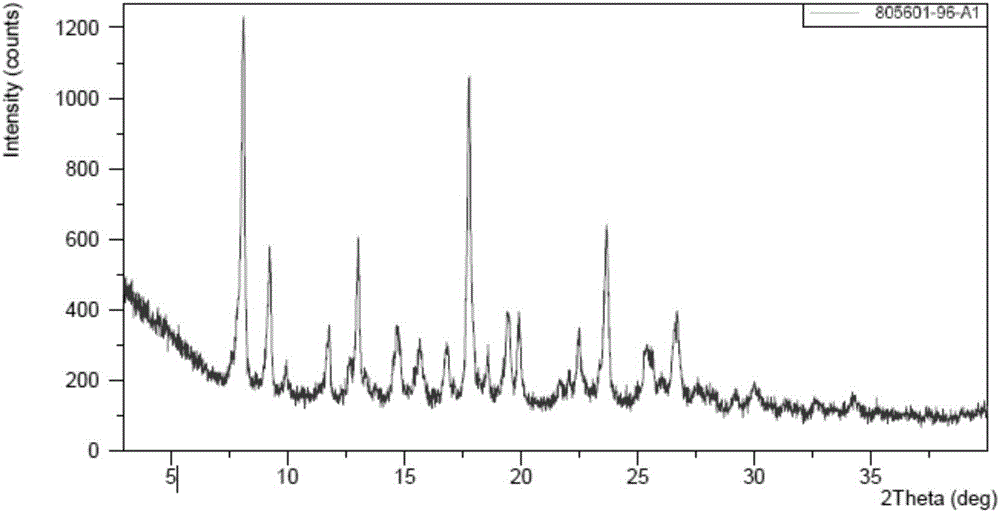
[0089] Example 1 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph B of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0090] Take 10g (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl)methyl Base) phenyl) (4-methylpiperidin-1-yl) ketone into a glass bottle, add 200mL ethyl acetate to the glass bottle and stir evenly, then add 3.5mL concentrated hydrochloric acid with a volume percentage of 37.5%, at room temperature Stir, react for 3 hours, and dry in vacuo to obtain (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl ) (3-hydroxyphenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride polymorph B.
[0091] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph B of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride is shown in figure 1 The peak information of its spectrum...
Embodiment 2
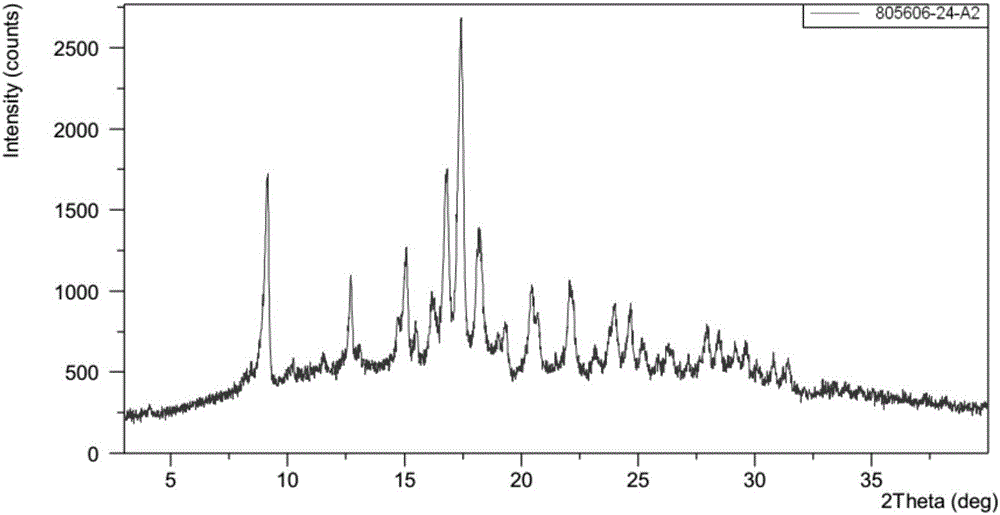
[0094] Example 2 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph C of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0095](4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)( 3-Hydroxyphenyl) methyl) phenyl) (4-methylpiperidin-1-yl) polymorph B of ketone dihydrochloride was used as a starting sample, and 100 mL of acetonitrile was added to make the polymorph The sample of substance B was completely dissolved to obtain a clear solution. The obtained solution was slowly volatilized at room temperature, and the collected solid was polymorph C.
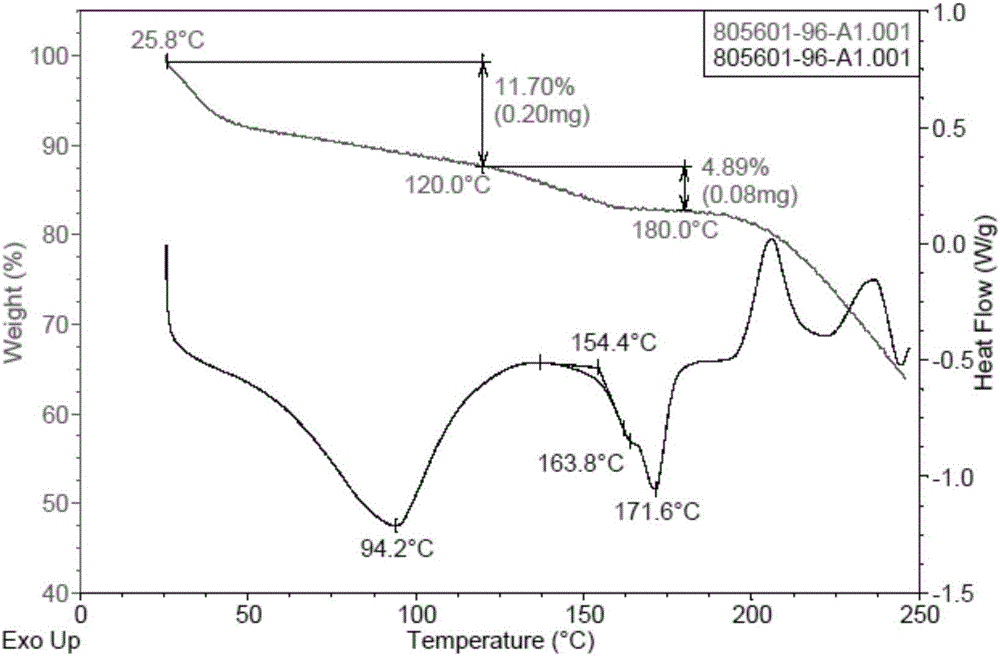
[0096] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy The XRPD pattern of polymorph C of phenyl)methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride is shown in image 3 The peak information of its spectrum is shown in Table 2. TGA and DSC diagrams of polymorph C Figure 4 shown by Figure 4 It can be se...
Embodiment 3
[0100] Example 3 (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxyphenyl) Preparation of polymorph C of methyl)phenyl)(4-methylpiperidin-1-yl)methanone dihydrochloride
[0101] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)( 3-hydroxyphenyl) methyl) phenyl) (4-methylpiperidin-1-yl) ketone dihydrochloride polymorph B as the starting sample, placed in an open glass bottle; add 100mL Acetonitrile to obtain a clear solution; continue to add mixed polymers (polycaprolactone, polyethylene glycol, polymethyl methacrylate, sodium alginate and hydroxyethyl cellulose mixed with quality); after fully stirring, place the sample Slowly volatilize at room temperature, and the obtained solid is polymorph C.
[0102] (4-((R)-((2S,5R)-4-(3-fluorobenzyl)-(2,5-dimethylpiperazin-1-yl)(3-hydroxy Phenyl) methyl) phenyl) (4-methylpiperidin-1-yl) XRPD figure, TGA and DSC figure of polymorph C of ketone dihydrochloride are respectively with image 3 and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com